Abstract
Purpose In the present MEG-study, power spectral analysis of oscillatory brain activity was used to compare resting state brain activity in both low-grade glioma (LGG) patients and healthy controls. We hypothesized that LGG patients show local as well as diffuse slowing of resting state brain activity compared to healthy controls and that particularly global slowing correlates with neurocognitive dysfunction. Patient and methods Resting state MEG recordings were obtained from 17 LGG patients and 17 age-, sex-, and education-matched healthy controls. Relative spectral power was calculated in the delta, theta, upper and lower alpha, beta, and gamma frequency band. A battery of standardized neurocognitive tests measuring 6 neurocognitive domains was administered. Results LGG patients showed a slowing of the resting state brain activity when compared to healthy controls. Decrease in relative power was mainly found in the gamma frequency band in the bilateral frontocentral MEG regions, whereas an increase in relative power was found in the theta frequency band in the left parietal region. An increase of the relative power in the theta and lower alpha band correlated with impaired executive functioning, information processing, and working memory. Conclusion LGG patients are characterized by global slowing of their resting state brain activity and this slowing phenomenon correlates with the observed neurocognitive deficits.
Similar content being viewed by others
Introduction
Low–grade glioma (LGG) patients constitute 25% [1] of the glioma patient population and have a survival of 5–10 years [2]. The optimal treatment for this patient group is still a matter of debate [3–5]. The first clinical feature of this disease is epilepsy in two-third of the patients [1, 4]. The majority of LGG patients also suffer from cognitive deficits, which tend to have a global character and cannot only be explained by the tumor localization alone [6, 7].
Higher cognitive functions depend on the integrated activity of several specialized brain areas. Functional imaging techniques such as electroencephalography (EEG) and magnetoencephalography (MEG) are used to characterize oscillatory activity in patients with neurodegenerative diseases, including Alzheimer’s [AD; 8–10] and Parkinson’s disease [PD; 11–13]. Oscillations are a feature of neuronal brain activity and the synchronization of the oscillatory activity (reflecting the interactions of neuronal activity) is a likely mechanism for neuronal communication. For an overview of the relation between oscillatory brain activity and neurocognitive function [see 14, 15].
Magnetoencephalography (MEG) is a relatively novel way to capture the dynamics of the electromagnetic fields within the brain, and combines excellent temporal and spatial resolution. Comparison of MEG in AD patients and healthy controls showed more delta and theta activity in the patient population especially in the temporo-parietal region [16]. Another study compared spectral power in four different groups (severe AD, moderate AD, Lewy Body dementia, and healthy controls) and showed that the healthy control population had the largest rhythmic activity in the alpha band. The moderate AD population and the patients with Lewy Body dementia had the rhythmic predominance in the pre-alpha frequency band (7–9 Hz), whereas the severe AD showed a shift towards the slow-band (3–7 Hz) [17]. In PD patients, MEG has been used by Kotini [18], who showed slowing of the alpha rhythm in the patient population compared to healthy controls, and Bosboom [19], who demonstrated slowing in the theta, beta, and gamma frequency band in non-demented PD patients with a further slowing (and involvement of delta and alpha band) in the demented PD patient population.
It is suggested that the increase in slow wave activity corresponds with neurocognitive deficits in AD as well as in PD [13, 16, 19, 20].
MEG-studies have previously been performed in brain tumor patients. Oshino [21] found an increase in delta and theta band activity especially in the vicinity of the tumor and in the surrounding regions of edema. In the majority of these patients, the increase correlated with the clinical symptoms of these patients, assessed with a routine neurological examination, but neurocognitive function were not formally investigated. Although the focal lesion in brain tumor patients contrasts with the generalized brain degeneration in AD and PD, there is evidence that brain tumors can cause functional disturbances in areas remote from the tumor [22–25]. De Jongh [22] found focal clusters of delta activity near the tumor area but also in the contralateral hemisphere. An evaluation of fast MEG waves in brain tumor patients showed dipoles located in the parietal and occipital area, whereas the tumors were located in the parietal, temporal, and frontal area [23]. In this study, these dipoles were found remote from the tumor area and even in the contralateral hemisphere. Bartolomei [24, 25] found differences in resting state functional connectivity in the same brain tumor population within several frequency bands when compared to healthy controls. Interestingly, in agreement with de Jongh’s results, these differences were not limited to the tumor area and were more obvious in those with a tumor in the left hemisphere.
Since higher cognitive functions are presumed to depend on the integrated activity of several specialized brain areas, it is suggested that neurocognitive deficits may have a stronger correlation with diffuse alterations in resting state brain oscillatory activity than focal abnormalities in the brain tumor population.
In the present MEG-study, power spectral analysis of oscillatory brain activity was used to compare resting state brain oscillatory activity in both LGG patients and healthy controls.
We hypothesize that (1) LGG patients show, in addition to the local abnormalities at the tumor site, global slowing of the resting state brain activity compared to healthy controls which will vary between patients with a tumor in the left or right hemisphere (2) changes in resting state brain oscillatory activity reflect an intermediate level between the impact of tumor and tumor-related treatment on the one hand (‘input’) and the neurocognitive deficits (‘output’) on the other hand and that diffuse slowing is correlated with higher neurocognitive dysfunction.
Materials and methods
Patients and controls
Twenty-three LGG patients were asked to participate in this study. Patients were eligible if: (a) they had a suspected or histologically confirmed LGG; (b) there was no radiological (confirmed by MR or CT scan) and/or clinical tumor progression in the previous 6 months; (c) they did not use medication possibly interfering with neurocognitive function, other than anti-epileptic drugs (AEDs).
Patients were recruited from the VU University Medical Center (VUmc) and the Academic Medical Center (AMC), both tertiary referral centres in Amsterdam for brain tumor patients, after the institutional ethical review boards of both participating hospitals approved the study protocol. Relatives of the patients were asked to participate as healthy controls. Healthy controls were eligible if they: (a) did not suffer from any neurological disease; (b) did not use any medication that might influence cognitive function. For patients who could not provide a healthy control participant, VU University Medical Center staff members were included.
Magnetoencephalography
MEG recordings were obtained using a 151-channel whole-head MEG system (CTF systems; Port Coquitlam, British Columbia, Canada) while participants were seated inside a magnetically shielded room (Vacuumschmelze GmbH, Hanau, Germany). Magnetic fields were recorded during a no-task, eyes-closed resting state. Metal artefacts were avoided as much as possible. A third-order software gradient [26] was used with a recording passband of 0.25–125 Hz and a sample frequency of 312.5 Hz. At the beginning, middle and end of each recording, the head position relative to the coordinate system of the helmet was recorded by leading small alternating currents through three head position coils attached to the left and right preauricular points and the nasion on the subject’s head. Head position changes up to approximately 1.5 cm during a recording condition were accepted.
For this study, 149 of the 151 channels could be used. MEG recordings were converted to ASCII files. From these ASCII files four artefact free epochs of 13 s per subject (4096 samples) were carefully selected by visual analysis by one of the authors (IB).
Magnetic field frequencies ranging from 0.5 to 80 Hz were recorded. The MEG data were digitally filtered off-line in the following frequency bands: delta (0.5–4 Hz), theta (4–8 Hz), lower alpha (8–10 Hz), upper alpha (10–13 Hz), beta (13–30 Hz), gamma (30–50 Hz).
The MEG channels were grouped into 10 distinct regions, respectively left and right frontal region, left and right temporal region, left and right parietal region, left and right occipital region and left and right central region, as shown in Fig. 1. Fast Fourier transformation was separately applied for every patient and control on the four epochs in the above mentioned frequency bands. The results of the four epochs were averaged for each participant and the mean relative power for each MEG region were used for the analysis.
Neurocognitive assessment
Patients and controls participated in neurocognitive assessment (see Table 1). The total duration of the assessment varied between 1 and 2 h. Individual patient’s test scores were converted to z-scores, using the means and standard deviations of the age-, sex- and education-matched healthy controls as a reference.
To reduce data, individual scores on these tests were summarized into six cognitive domains, namely information processing speed, psychomotor function, attention, verbal memory, working memory, and executive functioning. Construction of these domains has previously been reported [30], and was based on a Principal Component Analysis using Varimax rotation with Kaiser normalization performed on the z-scores of a large group of healthy controls [31]. The domains found are commonly used in neurocognitive practice and research.
Statistical analysis
Differences between both groups in the distribution of age, sex, and education were analyzed by means of chi-square tests. Mann–Whitney nonparametric U-tests were used to investigate whether patients’ standardised z-scores on neurocognitive tests in the overall measure of cognition differed significantly from healthy control z-scores.
Because of the non-normal distribution of the relative power, Mann–Whitney nonparametric U-tests were used to determine possible differences between the patient population and healthy controls.
To assess the association between relative power and cognition within the patient group, Spearman’s correlation coefficient (ρ) was calculated between the relative power in the different MEG regions and z-scores of the six neurocognitive domains for all separate frequency bands.
Results
Patient characteristics
From the initial patient group, six patients were excluded, four patients due to metal artefacts on the MEG measurements, one due to severe epileptic seizures, and one due to tumor progression at the time of registration. The final analyses were performed on a sample of 17 patients and 17 matched healthy control participants.
Due to the matching procedure, there were no significant differences between patients and healthy controls in age (M = 42.7, SD = 11.2 in patients, M = 42.6, SD = 12.7 in healthy controls, p = 0.99), and educational level (M = 5.2, SD = 1.8 in patients, M = 5.5, SD = 1.8 in healthy controls, p = 0.64). The male–female ratio between the two groups did not differ significantly (p = 0.37). Fourteen of the 17 LGG patients had a histologically confirmed LGG, clinically and radiologically stable for more than six months before inclusion, whereas the other 3 patients were suspected of having LGG, and stable for more than six months. The patients in our study were diagnosed several years ago (mean 8 years, range: 1–19 years). One year after MEG registration two of the three patients with suspected LGG were operated on because of increasing epilepsy frequency.
Eleven of those 16 patients underwent debulking, whereas 3 patients underwent a stereotactic biopsy and another two patients had an open biopsy. Of the 16 patients with a histologically confirmed LGG, the pathological diagnosis was grade II astrocytoma in ten patients, oligodendroglioma grade II in four patients and oligoastrocytoma grade II in two patients.
Seven of the seventeen patients underwent radiotherapy with prior chemotherapy in two patients (1 patient with 5 cycles of PCV and 1 patient with 2 cycles of PCV and 3 cycles of temozolomide). Eleven patients had left hemisphere tumors and 6 patients had right-sided tumors. The specific localisation of the tumor is shown in Table 2.
In the patient group, all but one patient used AED mono- or poly-therapy. Six of the 16 patients on AED were free of seizures, while the other 10 patients were still having seizures.
Differences between patient and healthy control group
Neurocognitive functioning
As expected, patients performed poorer than healthy controls on the neurocognitive test battery (controls M = 0.00). More specifically, patients showed a significantly lower psychomotor function (M = −0.50, SD = 0.75 versus SD = 0.55, p = 0.044), working memory capacity (M = −1.43, SD = 1.62 versus SD = 0.91, p = 0.002), information processing speed (M = −0.85, SD = 0.88 versus SD = 0.97, p = 0.018) and attention (M = −1.92, SD = 3.87 versus SD = 0.68, p = 0.003). Patients’ performance on the other two cognitive domains did not differ significantly from controls (Fig. 2).
Patients’ z-scores on the six neurocognitive domains and on total neurocognitive functioning. Note: * p < 0.05, ** p < 0.01. A = attention, EF = executive functioning, IPS = information processing speed, PF = psychomotor function, VM = verbal memory, WM = working memory. Performance is relative to that of age-, sex-, and education-matched healthy controls (represented by the 0-line). A higher score (i.e. approaching 0) means better performance
Spectral analysis
Global spectral analysis. The mean relative power of the LGG patient population showed a significant decrease (Mann–Whitney U, p = 0.034) in the gamma band (30–50 Hz) compared with healthy controls.
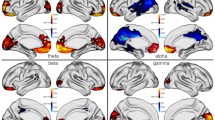
Spectral analysis of frequency bands within each MEG region. An increase (Mann–Whitney U; p = 0.014) in relative power in the LGG patients compared to the healthy controls was found in the left parietal region within the theta frequency band (4–8 Hz). A decrease in relative power was seen in the left and right central region (Mann–Whitney U; both p = 0.024) and left and right frontal region (Mann–Whitney U; both p = 0.005) of the LGG patient population compared to the healthy controls within the gamma band (30–50 Hz), as shown in Table 3 and Fig. 3.
The significant differences in relative power between the patient group and the healthy controls within the different frequency bands. Green area: significant higher relative power in the patient group compared to the healthy controls. Red area: significant lower relative power in the patient group compared to the healthy controls
Influence of tumor lateralization on relative power. Eleven patients had left hemisphere tumors and 6 patients had a tumor in the right hemisphere. Patients with a tumor in the left hemisphere showed an increase in theta activity in the left and right central region (Mann–Whitney U; respectively p = 0.001 and p = 0.014), left and right parietal region (respectively p = 0.004 and p = 0.029), and left and right temporal region (respectively p = 0.022 and p = 0.027) when compared to the healthy control population. Patients with left-sided tumors also showed a decrease in relative power in the gamma band in the left and right central region (respectively p = 0.023 and p = 0.048) and left and right frontal region (respectively p = 0.011 and p = 0.019).
Patients with a tumor in the right hemisphere showed a significant increase in relative power of delta activity in the right parietal region (Mann–Whitney U; p = 0.050) compared to the healthy control population. These patients also showed a decrease in relative power in the gamma band in the left and right frontal region (respectively p = 0.005 and p = 0.025), right temporal region (p = 0.042), and left and right occipital region (respectively p = 0.025 and p = 0.014), as shown in Table 4 and Fig. 4.
The significant differences in relative power between the patients with a tumor in the left or right hemisphere compared to the healthy controls within the different frequency bands. Green area: significant higher relative power in the patient group compared to the healthy controls. Red area: significant lower relative power in the patient group compared to the healthy controls
Since these results could be influenced by differences in tumor volume, we evaluated the tumor size of participating patients. We used T1-weigthed MRI and defined tumor size as the product of the two largest perpendicular diameters of tumor hypointensity [32]. We found no significant differences in the tumor size between left- and right-sided tumors.
Associations between patient’s neurocognitive functioning and relative power in distinct MEG regions
In the patient population, negative associations between neurocognitive functioning and relative power were found in the theta and lower alpha band. An increase in the relative power in the theta band within the left frontal (−0.606; p = 0.010) and the right frontal region (−0.490; p = 0.046) was correlated with a poorer executive functioning. In the same frequency band (4–8 Hz), an increase in theta band power was correlated with poorer information processing within the left central and left frontal region (respectively −0.515; p = 0.034 and −0.592; p = 0.012). Within the lower alpha band in the right temporal region, an increase in relative power was correlated with a disturbed working memory (−0.508; p = 0.037).
Discussion
The primary goal of the present study was to evaluate whether LGG patients show (in addition to the well known MEG slowing around the tumor) diffuse slowing in resting state brain activity. The secondary goal of the study was to investigate whether this slowing is correlated with neurocognitive dysfunction. By means of MEG-registrations we have demonstrated that LGG patients have slowing of the resting state brain activity when compared to healthy controls. The decrease in relative power was mainly found in the gamma frequency band in the bilateral frontocentral MEG regions. Regarding the low frequency bands, an increase in relative power was found in the theta frequency band in the left parietal region. Correlations of neurocognitive functioning with the relative power in the patient population showed clear associations in the lower alpha and theta band, increased slowing correlating with poorer performance.
We chose to use the relative power instead of the absolute power, because relative power is less influenced by the distance between the MEG sensor and the underlying neural populations. The distance is variable due to head position in the helmet and the thickness of the skull. Lower variance of power values in subject groups are expected by using the relative power instead of the absolute power. A second reason for us to use this method is the fact that by using this method we are able to compare our results with those of other patient cohorts at our MEG center [19, 20]. This includes comparisons with other brain tumor data sets but also the comparison of our results with neurodegenerative diseases (including AD and Parkinson’s disease (PD)). This will give us an opportunity to learn more about differences in resting state oscillatory brain activity in the different diseases and at different disease stages. A limitation of this method of analysis is that an increase in one frequency band can cause a decrease in another band, although this might not be the observed pattern in absolute measures.
In previous studies, MEG has been used for power analysis in patients with neurodegenerative diseases, such as AD and PD. They showed similar results, namely a rhythmic predominance in the lower frequency bands, as was found in our study with LGG patients [16–20]. There are only few studies performed in brain tumor patients, however.
De Jongh [22] demonstrated that both a higher tumor malignancy and larger tumor volume were associated with higher signal powers in the delta band. In our study, we did not find increased oscillatory brain activity in the delta band. It might be that the conflicting results are due to differences in methodology of both studies. In contrast with De Jongh’s study [22], we analysed the relative power, which means that the power is influenced by the power in the other frequency bands and therefore group differences in a specific frequency band could results from changes in that specific band or in the absolute power of the other frequency bands. Furthermore, we evaluated diffuse delta activity instead of clusters of dipoles in the vicinity of the tumor. Apart from these technical differences, De Jongh’s patient population consisted of a more heterogeneous group of brain tumor patients. They found a higher delta power in the high-grade gliomas compared to those patients with a low-grade glioma and a delta signal power decrease after surgery. Our patient population consisted exclusively of patients with a low-grade glioma, and all but three MEG registrations were done after surgery, which might explain the lack of changes in the delta band in the present study. It also suggests that changes in the gamma and theta band may be more sensitive indicators of brain dysfunction in LGG.
In another study of De Jongh [23], the authors found focal clusters of fast MEG waves outside the tumor area and even in the contralateral hemisphere. They concluded that the asymmetry possibly reflects features of normal background activity, since asymmetry has already been demonstrated in healthy subjects for the alpha activity which is usually dominant in the right hemisphere. Since De Jongh used a single dipole analysis instead of our analysis of diffuse power changes and, again, analysed a group of patients with diverse brain tumors, it is difficult to compare the results of both studies.
Oshino and colleagues [21] used synthetic aperture magnetometry (SAM) to analyze oscillatory activity recorded by the MEG. SAM is an alternative method investigating the distribution of reconstructed sources over different cortical regions. In contrast to other source localization methods, beamforming does not rely on averaging and therefore allows analysis of evoked and induced brain activity. In a group of 15 patients with various types of primary or secondary brain tumors, they found an increase in delta and theta band activity especially in the vicinity of the tumor and in the surrounding regions of edema. In the majority of these patients, the increase in the delta band correlated well with the clinical symptoms, assessed with a routine neurological examination. The results were not correlated with neurocognitive functioning because they stated that there is much individual variation in brain activity related to neurocognitive function and they expected it difficult and sometimes impossible for these patients to undergo the neurocognitive battery. In contrast to their results, we only found an increase in theta activity and decrease in the gamma frequency band. As mentioned before we analyzed the relative power and also our patient population with only LGG patients is not comparable to the patients with various brain tumors in the study of Oshino. Although, different methods to reconstruct sources can show significant discrepancies, we also found spectral changes in the contralateral hemisphere.
By evaluating separately those patients with a tumor localisation in the left or the right hemisphere, we found more significant differences in patients with a tumor in the left hemisphere compared to healthy controls. In agreement with our previous study [24], in which we evaluated the functional connectivity (functional connectivity is a statistical correlation between time series of brain activity recorded over distinct regions which are assumed to reflect interactions between the brain regions) in patients with diverse primary brain tumors, we also found that patients with a tumor in the left hemisphere showed more differences in this functional connectivity compared to those with right-sided tumors.
Why those patients with a tumor in the left hemisphere do show more differences in oscillatory activity is not known. It is possible that the left hemisphere (usual the dominant hemisphere) acts differently to the tumor compared to those located in the right hemisphere. It is also possible that patients with a tumor in the left hemisphere are in a different stage of their disease compared to those with a tumor in the right hemisphere. Since our sample size is rather small, this could be reflected on the observed results. On the other hand is it possible that our results reflect hemispheric asymmetry which has been found in the healthy population previously. Gootjes et al. [33] found hemispheric differences in functional connectivity in healthy young adults during resting state.
The neurocognitive domains of psychomotor function, working memory, information processing speed and attentional tasks were impaired in LGG patients. These results corroborate previous studies indicating a decline in neurocognitive functioning in LGG patients [6, 34–38]. Several research groups have suggested that the increase in slow wave activity corresponds with neurocognitive deficits in AD and PD. Fernandez [16] found right parietal delta activity to be significantly associated with variability in the Cambridge Examination for Mental Disorders in the elderly (CAMCOG) score, whereas the left temporal theta activity predicted the variability of Mini Mental State Examination (MMSE) results. Sinanovic [13] found a positive correlation between diffuse changes in spectral power and MMSE scores in the PD patients with dementia. Bosboom [19] found a negative correlation in the non-demented PD patients between theta power and CAMCOG scores in both occipital and right temporal region but expected these associations, given the large number of relations that were analyzed, likely to be caused by coincidence. In the demented PD group they found no significant correlations. Stoffers [20] showed a negative association between lower alpha power and the performance on perseveration-related tasks in the non-demented PD patients.
Evidently, this study has its limitations. First of all, the patient group is rather small as mentioned before. Secondly, although our patient population consisted of only LGG patients, they did not all receive the same treatment. In our study we hypothesized changes in oscillatory brain activity to be the intermediate between the impact of the tumor and its treatment on the one hand and the neurocognitive deficits as the output on the other hand. This study showed that irrespective of the different treatment options, changes in oscillatory brain activity can be found in brain tumor patients which is associated with neurocognitive function. To get informed on the influence of the different tumor treatments on brain activity and its relation with neurocognitive function is very interesting. A longitudinal study of these effects (e.g. surgery, radiotherapy and chemotherapy) on brain activity is currently under way. The goal of that study is to explore correlations between changes in neurocognitive function and changes in functional brain dynamics during the disease course.
Our study is the first to correlate the relative power with neurocognitive functioning in brain tumor patients. The observed correlations were quite strong and showed that an increased activity in the theta and lower alpha band is correlated with impaired executive functioning, information processing and working memory. This is a first step in unraveling the underlying mechanisms of neurocognitive dysfunction in brain tumor patients. MEG power analysis gives us an interesting tool to assess functional alterations in the patient’s brain in the course of disease and to evaluate its relationship with neurocognitive functioning.
References
Shaw EG, Scheithauer BW, O’Fallon JR (1993) Management of supratentorial low-grade gliomas. Oncology 7(7):97–111
Shaw EG, Daumas-Duport C, Scheithauer BW et al (1989) Radiation therapy in the mangement of low-grade supratentorial astrocytomas. J Neurosurg 70:853–861
Reijneveld JC, Sitskoorn MM, Klein M et al (2001) Cognitive status and quality of life in patients with suspected versus proven low-grade gliomas. Neurology 56:618–623
Wessels PH, Weber WE, Raven G et al (2003) Supratentorial grade II astrocytoma: biological features and clinical course. Lancet Neurol 7(2):395–403
van den Bent MJ, Afra D, de Witte O et al (2005) Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet 336:985–990
Klein M, Heimans JJ, Aaronson NK et al (2002) Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet 360(9343):1361–1368
Taphoorn MJ, Klein M (2004) Cognitive deficits in adult patients with brain tumours. Lancet Neurol 3(3):159–168
Dierks T, Ihl R, Frolich L, Maurer K (1993) Dementia of the Alzheimer type: effects on the spontaneous EEG described by dipole sources. Psychiatr Res 50(3):151–162
Jelic V, Johansson SE, Almkvist O et al (2002) Quantitative electroencephalography in mild cognitive impairment: longitudinal changes and possible prediction of Alzheimer’s disease. Neurobiol Aging 21(4):533–540
Babiloni C, Binetti G, Casseta E et al (2004) Mapping distributed sources of cortical rythms in mild Alzheimer’s disease. A multicentric EEG study. Neuroimage 22:57–67
Soikkeli R, Partanen J, Soininen H et al (1991) Slowing of EEG in Parkinson’s disease. Electroencephalogr Clin Neurophysiol 79(3):159–165
Neufeld MY, Blumen S, Aitkin I, Parmet Y, Korczyn AD (1994) EEG frequency analysis in demented and nondemented parkinsonian patients. Dementia 5(1):23–28
Sinanovic O, Kapidzic A, Kovacevic L et al (2005) EEG frequency and cognitive dysfunction in patients with Parkinson’s disease. Med Arh 59(5):286–287
Schnitzler A, Gross J (2005) Normal and pathological oscillatory communication in the brain. Nature reviews neuroscience 6:285–296
Uhlhaas PJ, Singer W (2006) Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathofysiology. Neuron 52:155–168
Fernandez A, Maestu F, Amo C et al (2002) Focal temporoparietal slow activity in Alzheimer’s disease revealed by magnetoencephalography. Biol Psychiatry 52:764–770
Franciotti R, Iacono D, Della Penna S et al (2006) Cortical rythms reactivity in AD, LBD, and normal subjects: a quantitative MEG study. Neurobiol aging 27(8):1100–1109
Kotini A, Anninos P, Adamopoulos A et al (2005) Low-frequency MEG activity and MRI evaluation in Parkinson’s disease. Brain Topography 18(1):59–63
Bosboom JLW, Stoffers D, Stam CJ et al (2006) Resting state oscillatory brain dynamics in Parkinson’s disease: a MEG study. Clin Neurophysiol 117:2521–2531
Stoffers D, Bosboom JLW, Deijen JB et al (2007) Slowing of oscillatory brain activity is a stable characteristic of Parkinson’s disease without dementia. Brain 130:1847–1860
Oshino S, Kato A, Wakayama A et al (2007) Magnetoencephalographic analysis of cortical oscillatory activity in patients with brain tumors: synthetic aperture magnetography (SAM) functional imaging of delta band activity. Neuroimage 34:957–964
de Jongh A, Baayen JC, de Munck JC et al (2003) The influence of brain tumor treatment on pathological delta activity in MEG. Neuroimage 20:2291–2301
de Jongh A, de Munck JC, Baayen JC et al (2003) Localization of fast MEG waves in patients with brain tumors and epilepsy. Brain topography 15(3):173–179
Bartolomei F, Bosma I, Klein M et al (2006) How do brain tumors alter functional connectivity? A magnetoencephalography study. Ann Neurol 59(1):128–138
Bartolomei F, Bosma I, Klein M et al (2006) Disturbed functional connectivity in brain tumour patients: evaluation by graph analysis of synchronization matrices. Clin Neurophysiol 117(9):2039–2049
Vrba J, Anderson G, Betts K (1999) 151-Channel whole-cortex MEG system for seated or supine positions, recent advances in biomagnetism. Tohoku University Press, Sendai, pp 93–96
Lezak MD (1995) Neuropsychological assessment. Oxford University Press, New York
Benton AL (1968) Differential behavioral effects in frontal lobe disease. Neuropsychologia 6:53–60
Houx PJ, Jolles J (1994) Vulnerability factors for age-related cognitive decline. In: Jensen KF (ed) The vulnerable brain and environmental risks, vol 3. Plenum Press, New York, pp 25–41
Klein M, Postma TJ, Taphoorn MJ et al (2003) The prognostic value of cognitive functioning in the survival of patients with high-grade glioma. Neurology 61(12):1796–1798
Bosma H, van Boxtel MP, Ponds RW et al (2000) Pesticide exposure and risk of mild cognitive dysfunction. Lancet 356(9233):912–913
Vos MJ, Uitdehaag BM, Barkhof F et al (2003) Interobserver variability in the radiological assesment of response to chemotherapy in glioma. Neurology 61(12):826–830
Gootjes L, Bouma A, Van Strien JW, Scheltens P, Stam CJ (2006) Attention modulates hemispheric differences in functional connectivity; Evidence from MEG recordings. Neuroimage 30:245–253
Hochberg FH, Slotnick B (1980) Neuropsychologic impairment in astrocytoma survivors. Neurology 30(2):172–177
Taphoorn MJ, Heimans JJ, Snoek FJ et al (1992) Assessment of quality of life in patients treated for low-grade glioma: a preliminary report. J Neurol Neurosurg Psychiatry 55(5):372–376
Taphoorn MJ, Schiphorst AK, Snoek FJ et al (1994) Cognitive functions and quality of life in patients with low-grade gliomas: the impact of radiotherapy. Ann Neurol 36(1):48–44
Meyers CA, Brown PD (2006) Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol 24(8):1305–1309
Shaw EG, Rosdhal R, D’Agostino RB et al (2006) Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. J Clin Oncol 24(9):1415–1420
Acknowledgments
We thank Dr. L.J.A. Stalpers (radiation oncologist) and J.C. Baaijen (neurosurgeon) for their help in recruiting patients; J. Verbunt, P.J. Ris, I. Manshanden, G. de Vos for technical assistance; Mrs. E. van Deventer for providing relevant literature.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by Schering Plough Nederland and the Kapteijnfonds.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Bosma, I., Stam, C.J., Douw, L. et al. The influence of low-grade glioma on resting state oscillatory brain activity: a magnetoencephalography study. J Neurooncol 88, 77–85 (2008). https://doi.org/10.1007/s11060-008-9535-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-008-9535-3