Abstract
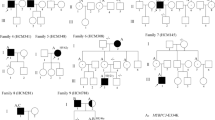
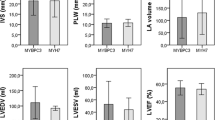
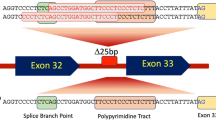
The aim of the current study was to determine the frequency of mutations in the beta-myosin heavy chain gene (MYH7) in a cohort of hypertrophic cardiomyopathy (HCM) and dilated cardiomyopathy (DCM) and their families, and to investigate correlations between genotype and phenotype. About 130 consecutive patients diagnosed with HCM or DCM (69 with HCM and 61 with DCM) attending the cardiology clinic of Post Graduate Institute of Medical Education and Research were screened for mutations in the MYH7 gene. The control group for genetic studies consisted of 100 healthy subjects. We report 14 mutations in 6 probands (5 probands in HCM and 1 proband in DCM) and their family members. Out of these 6 mutations, 3 are new and are being reported for the first time. One known mutation (p.Gly716Arg) was found to be “de novo” which resulted in severe asymmetric septal hypertrophy (31 mm) and resulted in the sudden cardiac death (SCD) of the proband at the age of 21 years. Further, a DCM causing novel mutation p.Gly377Ser was identified which resulted in the milder phenotype. The present study shows that there is genetic and phenotypic heterogeneity of cardiomyopathies in Indian population. Further, the location and type of mutation in a given sarcomeric gene determines the severity and phenotypic plasticity in cardiomyopathies.








Similar content being viewed by others
References
Koppole S, Smith JC, Fischer S (2006) Simulations of the myosin II motor reveal a nucleotide-state sensing element that controls the recovery stroke. J Mol Biol 361:604–616. doi:10.1016/j.jmb.2006.06.022
Van Driest SL, Jaeger MA, Ommen SR, Will ML, Gersh BJ, Tajik AJ, Ackerman MJ (2004) Comprehensive analysis of the beta-myosin heavy chain gene in 389 unrelated patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 44:602–610. doi:10.1016/j.jacc.2004.04.039
Havndrup O, Bundgaard H, Andersen PS, Larsen LA, Vuust J, Kjeldsen K, Christiansen M (2001) The Val606Met mutation in the cardiac beta-myosin heavy chain gene in patients with familial hypertrophic cardiomyopathy is associated with a high risk of sudden death at young age. Am J Cardiol 87:1315–1317. doi:10.1016/S0002-9149(01)01532-6
Fananapazir L, Epstein ND (1994) Genotype–phenotype correlations in hypertrophic cardiomyopathy. Insights provided by comparisons of kindreds with distinct and identical beta-myosin heavy chain gene mutations. Circulation 89:22–32
Nakajima-Taniguchi C, Azuma J, Nagata S, Kishimoto T, Yamauchi-Takihara K (1995) A missense mutation in the beta-myosin heavy chain gene in a Japanese patient with hypertrophic cardiomyopathy. Jpn Circ J 59:833–837
Hwang TH, Lee WH, Kimura A, Satoh M, Nakamura T, Kim MK, Choi SK, Park JE (1998) Early expression of a malignant phenotype of familial hypertrophic cardiomyopathy associated with a Gly716Arg myosin heavy chain mutation in a Korean family. Am J Cardiol 82:1509–1513. doi:10.1016/S0002-9149(98)00695-X
Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, Benaiche A, Isnard R, Dubourg O, Burban M, Gueffet JP, Millaire A, Desnos M, Schwartz K, Hainque B, Komajda M (2003) Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation 107:2227–2232. doi:10.1161/01.CIR.0000066323.15244.54
Tanjore RR, Sikindlapuram AD, Calambur N, Thakkar B, Kerkar PG, Nallari P (2006) Genotype–phenotype correlation of R870H mutation in hypertrophic cardiomyopathy. Clin Genet 69:434–436. doi:10.1111/j.1399-0004.2006.00599.x
Bashyam MD, Savithri GR, Gopikrishna M, Narasimhan C (2007) A p.R870H mutation in the beta-cardiac myosin heavy chain 7 gene causes familial hypertrophic cardiomyopathy in several members of an Indian family. Can J Cardiol 23:788–790
Kimura A, Harada H, Park JE, Nishi H, Satoh M, Takahashi M, Hiroi S, Sasaoka T, Ohbuchi N, Nakamura T, Koyanagi T, Hwang TH, Choo JA, Chung KS, Hasegawa A, Nagai R, Okazaki O, Nakamura H, Matsuzaki M, Sakamoto T, Toshima H, Koga Y, Imaizumi T, Sasazuki T (1997) Mutations in the cardiac troponin I gene associated with hypertrophic cardiomyopathy. Nat Genet 16:379–382. doi:10.1038/ng0897-379
Mogensen J, Murphy RT, Kubo T, Bahl A, Moon JC, Klausen IC, Elliott PM, McKenna WJ (2004) Frequency and clinical expression of cardiac troponin I mutations in 748 consecutive families with hypertrophic cardiomyopathy. J Am Coll Cardiol 44:2315–2325. doi:10.1016/j.jacc.2004.05.088
Mogensen J, Kubo T, Duque M, Uribe W, Shaw A, Murphy R, Gimeno JR, Elliott P, McKenna WJ (2003) Idiopathic restrictive cardiomyopathy is part of the clinical expression of cardiac troponin I mutations. J Clin Invest 111:209–216
Cuda G, Fananapazir L, Zhu WS, Sellers JR, Epstein ND (1993) Skeletal muscle expression and abnormal function of beta-myosin in hypertrophic cardiomyopathy. J Clin Invest 91:2861–2865. doi:10.1172/JCI116530
Liu SX, Hu SJ, Sun J, Wang J, Wang XT, Jiang Y, Cai J (2005) Characteristics of the beta myosin heavy chain gene Ala26Val mutation in a Chinese family with hypertrophic cardiomyopathy. Eur J Intern Med 16:328–333. doi:10.1016/j.ejim.2005.02.008
Nishi H, Kimura A, Harada H, Koga Y, Adachi K, Matsuyama K, Koyanagi T, Yasunaga S, Imaizumi T, Toshima H (1995) A myosin missense mutation, not a null allele, causes familial hypertrophic cardiomyopathy. Circulation 91:2911–2915
Van Driest SL, Ackerman MJ, Ommen SR, Shakur R, Will ML, Nishimura RA, Tajik AJ, Gersh BJ (2002) Prevalence and severity of “benign” mutations in the beta-myosin heavy chain, cardiac troponin T, and alpha-tropomyosin genes in hypertrophic cardiomyopathy. Circulation 106:3085–3090. doi:10.1161/01.CIR.0000042675.59901.14
Acknowledgments
TSR, SA and TSA received SRF from ICMR, India.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rai, T.S., Ahmad, S., Bahl, A. et al. Genotype phenotype correlations of cardiac beta-myosin heavy chain mutations in Indian patients with hypertrophic and dilated cardiomyopathy. Mol Cell Biochem 321, 189–196 (2009). https://doi.org/10.1007/s11010-008-9932-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9932-0