Abstract
Sleep problems are prevalent among autistic children and children with Rare Genetic Neurodevelopmental Disorders (RGND). Behavioral interventions are commonly used to treat sleep problems, with most involving extinction. While effective, the occurrence of a response burst (i.e., temporary worsening of the behavior) can result in a temporary increase in parent and child distress, and negatively affect treatment adherence. Thus, it is important to develop less restrictive treatment options. This study used a single case multiple baseline design to investigate the effectiveness and acceptability of less restrictive behavioral interventions (i.e., specifically excluding extinction) for sleep problems in ten autistic children and children with RGND (M = 7.3 years). Results demonstrated a reduction in sleep disturbance including unwanted bed-sharing, night wakings and sleep onset delay for 3/3, 5/5 and 6/7 children respectively, which were maintained at follow-up. Interventions were rated favorably by parents. The clinical implications of these findings are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Neurodevelopmental disorders (NDD) are a group of conditions characterized by persistent developmental delays, resulting in substantial functional limitations. Such impairments affect at least one developmental domain, including cognitive functioning, communication, social skills and/or motor ability, and must manifest prior to 22 years of age (Odom et al., 2007; Thambirajah, 2011; Volkmar et al., 2014). This category of disorders includes Autism, Down syndrome, Attention Deficit Hyperactivity Disorder (ADHD) and Rare Genetic Neurodevelopmental Disorders (RGND). RGND include those disorders which affect < 1 in 2000 people (European Commission, 2021); are a result of genetic alterations; and are associated with intellectual and developmental delay (McLay et al., 2019a, b; Woodford et al., 2021). Examples of RGND include Rett syndrome, Prader-Willi syndrome, and Fragile-X syndrome. In addition to developmental delay, those with NDD often exhibit phenotypic physiological and intellectual impairments that can result in behavior difficulties, including sleep problems.
Issues with the initiation and maintenance of sleep are the most frequently reported sleep problems experienced by children with NDD (Krakowiak et al., 2008; Richdale & Schreck, 2009) with prevalence rates ranging from 50%–80% (Angriman et al., 2015; Krakowiak et al., 2008; Kronk et al., 2010; Moss et al., 2014). Unwanted bed-sharing (i.e., co-sleeping) is also common (McLay et al., 2019a, 2019b). Without effective treatment, sleep problems in children with NDD are unlikely to resolve, resulting in profound long-term effects on the daytime behavior, adaptive functioning, and well-being of children and their families (Goldman et al., 2012; Kronk et al., 2010; Mörelius & Hemmingsson, 2014; Shochat et al., 2014).
Sleep problems in those with NDD are thought to be the result of a combination of biopsychosocial factors including impaired melatonin regulation (Woodford et al., 2021), co-occurring medical (e.g., epilepsy, sleep-related breathing disorders and asthma) (Ghanizadeh & Faghih, 2011; Parish, 2009), and psychiatric disorders (e.g., anxiety, depression and ADHD; Konjarski et al., 2018). Furthermore, environmental and behavioral factors (e.g., device use, an inconsistent and/or stimulating bedtime routine and parental reinforcement of sleep-interfering behaviors) also play a role in sleep problems (Blampied, 2013; Blampied & France, 1993; France & Blampied, 1999).
Given the bio-behavioral etiologies of sleep problems in children with NDD, it is perhaps unsurprising that most empirically supported interventions for these children are pharmacological (e.g., exogenous melatonin; Davis et al., 2018; McLay et al., 2021a, b, c) and behavioral (i.e., based on the principles of Applied Behavior Analysis; Carnett et al., 2020; McLay et al., 2021; Rigney et al., 2018). Behavioral sleep interventions typically include modifications to the child’s sleep environment and assisting parents to establish effective stimulus control for bed-preparation and sleep onset, and manage reinforcement contingencies for sleep-interfering and -facilitative behaviors. A common element in contingency management is behavioral extinction, involving elimination of reinforcers for sleep interfering behaviors (Allen et al., 2013; Jin et al., 2013; McLay et al., 2017, 2019a, b; Piazza et al., 1997; Weiskop et al., 2005).
The multifaceted etiology of sleep problems means that selection of any intervention is a comprehensive and complex clinical process. In the selection of behavioral interventions, this process can be informed by Functional Behavioral Assessment (FBA; Jin et al., 2013; McLay et al., 2019a, b). FBA is commonly used to identify environmental factors (antecedents) and contingencies of reinforcement (consequences) that elicit and maintain the problem behavior (Blampied, 2013). FBA outcomes are then used to inform the development of individualized intervention procedures, directly targeting these factors. Within the sleep context, FBA-informed interventions may focus on antecedent modifications such as changes to the discriminative stimuli associated with sleep (e.g., sleep onset location); establishing motivating operations that promote the independent onset and maintenance of sleep, for example, increasing homeostatic sleep pressure (i.e., the need for sleep) and consequently the reinforcing value of sleep through delayed bedtime, sleep restriction and eliminating daytime naps (Laraway et al., 2003); modifications to sleep hygiene; and teaching strategies that support autonomous sleep (e.g., deep breathing and progressive muscle relaxation). In addition, FBA guides any modification of contingencies of reinforcement. This includes providing reinforcement for sleep-facilitative behaviors, and/or the withdrawal of reinforcement (e.g., parent attention, access to preferred activities or items) for sleep interfering behaviors, also known as extinction (McLay, France, Blampied, van Deurs et al., 2021; McLay et al., 2019a, b; Weiskop et al., 2005).
A considerable amount of research has evaluated the effectiveness of FBA-based behavioral interventions for sleep in autistic children (Carnett et al., 2020; Jin et al., 2013; McLay et al., 2017; McLay, France, Blampied, van Deurs et al., 2021; McLay et al., 2019a, 2019b; Sanberg et al., 2018; van Deurs et al., 2021). However, research with those with RGND is extremely limited, with McLay et al. (2019a, b) locating only two studies that have evaluated FBA-based behavioral interventions for sleep in children with Fragile-X and Prader-Willi syndromes (Didden et al., 1998; Weiskop et al., 2005). Furthermore, there is some controversy among parents and professional communities about the use of behavioral extinction procedures, a debate centred around both philosophical and practical considerations. Practically speaking, implementing extinction and modified extinction procedures, may result in a response burst (i.e., a temporary increase in targeted behavior) at the outset of intervention (Blunden et al., 2016; Etherton et al., 2016; Middlemiss & Kendall-Tackett, 2014). This is the result of the withdrawal of reinforcement that previously maintained the targeted behavior, and paradoxically, makes the behavior worse as a prelude to positive behavior change as the process of extinction reduces the behaviour to low or zero frequency. In the case of sleep interventions, this can result in an increase in the frequency and intensity of crying, protesting, getting out of bed and other behaviors that have previously resulted in reinforcement (Blunden et al., 2016; France et al., 1996). This intensification in behavior can be difficult to ignore and is often intolerable to parents. As a result, many are unable to adhere to the treatment programme, resulting in unintentional reinforcement of the sleep interfering behavior, and subverting the extinction process and its desirable effects. Furthermore, the use of extinction-based procedures often requires high levels of expert guidance and support, the likes of which is not readily available in many communities. Philosophically, debate is often centred around fears about the stress that children may experience as a result of the extinction process (Etherton et al., 2016); understandings regarding attachment theory and its emphasis on the importance of responsive parenting (e.g. see Ainsworth, 1969), often perceived to be at odds with the extinction procedure (i.e. withdrawal or minimization of responding); and any parental distress that may be experienced during the extinction process (Whittall et al., 2019).
Attempts to ameliorate the occurrence of a response burst have long been investigated (Blunden et al., 2016; Kuhn et al., 2020). It is reasonable to expect that the use of modified extinction procedures in which reinforcement is gradually faded (e.g., graduated extinction, faded parental presence) or use of antecedent-based modifications in conjunction with extinction-based procedures, may reduce the likelihood of a response burst (Kuhn et al., 2020; McLay et al., 2019a, b; Roberds-Roach et al., 2012). Moreover, it may be possible to effectively treat sleep problems without using extinction procedures at all. For example, interventions that include modification of children’s sleep/wake schedules, may increase homeostatic sleep pressure during the night, reducing the likelihood of night waking (NW) and in turn, the need to implement extinction-based treatments.
The “less restrictive alternative” doctrine advises therapists to commence treatment using minimally sufficient and less aversive procedures (Johnston & Sherman, 1993; Kazdin, 2013). In the context of sleep, less restrictive interventions are those that are acceptable to families and that are considered likely to produce relatively less parent and child distress compared to other interventions. This may include interventions that do not reduce rates of reinforcement, do not require parental ignoring of the child and may produce less crying and evidence of child distress (i.e., alternatives to, or modifications of extinction; France & Blampied, 2005). Less restrictive interventions may be particularly important among parents of children with NDD who often experience medical complexity (e.g., gastrointestinal issues, epilepsy, asthma, feeding tubes, hypotonia) and where the need to avoid distress, and maintain parent responding, is a necessary safety measure (Stores, 2016).
To date, there has been little research that has directly adopted the less restrictive principle in designing behavioural sleep interventions in children with NDD (Van Deurs et al., 2021). Furthermore, we know little about whether there are differences in the types of NDD that may impact response to less restrictive interventions. It is possible that a child’s response to behavioral sleep interventions varies according to diagnosis, owing to the differences in causal mechanisms. For example, there are high rates of co-occurring biological conditions and neuro-endocrine abnormalities in children with RGND (Alabaf et al., 2019) which may impact the severity, type and topography of sleep problems. Such differences may also impact the effectiveness of behavioral interventions for sleep and parent perceptions of the acceptability of these interventions.
The purpose of this study is to (a) investigate the effectiveness of less restrictive alternatives to extinction-based procedures in the treatment of sleep problems in autistic children and children with RGND; (b) to investigate the maintenance of treatment effects following such treatment; (c) to evaluate whether improvement in sleep results in any collateral benefit to children’s daytime behavior and parent and child wellbeing; and (d) to assess parental acceptability of less restrictive intervention approaches. In addition, treatments, outcomes and acceptability were compared across autistic and RGND participants to see, in a preliminary way, if there was any indication of differential patterns associated with diagnostic status.
Method
Participants and Recruitment
This research was approved by the relevant university Human Ethics Committee. Informed consent was obtained from all parent participants and, consent or assent was provided by most children. Participants were recruited from throughout New Zealand via service providers for children with NDD and their families and via the professional networks of the research team. Participants were recruited for two different research studies focused on investigating the effectiveness of behavioral interventions for sleep problems in autistic children or children with RGND.
Participants met the following inclusion criteria: (a) between 2 and 21 years of age; (b) a formal diagnosis of autism or RGND as verified by a Psychiatrist, Psychologist and/or genetic testing (the latter for RGND); and (c) had parent-reported behavioral sleep disturbances (e.g., sleep onset delay, frequent and/or prolonged NW, bedtime resistance), later confirmed via sleep assessment. Children were excluded if they had medical conditions that were a contraindicator to behavioral intervention, or the timing and/or dose of medication administration was unstable at any phase. Ten children and adolescents (four female and six male) between the ages of 4 and 19 years met criteria for inclusion in the study. Five had a primary diagnosis of autism and five had a RGND. A summary of participant characteristics is presented in Table 1.
Study Design
Treatment outcomes were evaluated using a single-case non-concurrent multiple baseline across participants design (Barlow et al., 2009; Christ, 2007) with random allocation to baseline lengths. Individualized FBA-informed treatment plans were implemented across participants.
Settings
Assessments were undertaken in person in the family home or at the University of Canterbury clinic (6/10 participants), or via Zoom (4/10 participants). Interventions were implemented by parents, in the family home, with the support of the therapy team.
Assessment Measures
Child Communication Level
The Vineland Adaptive Behavior Scale – 3rd Edition Parent/Caregiver Rating Form (VABS-III; Sparrow et al., 2016) was administered during the assessment process. The VABS-III is a parent-report measure of children’s adaptive behavior with 120 items grouped into four subscales: Communication, Daily Living, Motor Skills and Socialization. The Communication subscale of the VABS-III was completed pre-treatment to assess children’s language abilities (completed by 8/10 parents; see Table 1 for VABS-III data).
Clinical Interview
Clinical interviews were conducted by a New Zealand registered Psychologist or Intern Psychologist. The Sleep Assessment Treatment Tool (SATT; Hanley, 2005); a semi-structured assessment tool, was used during the clinical interview to gather information about: (a) the history of the child’s sleep problem(s); (b) the type and topography of the sleep problem(s); (c) disruptive bedtime behaviors; (d) sleep hygiene practices and the nature of the sleep environment; (e) sleep/wake schedules; (f) any antecedent and consequence variables related to the sleep problem; and (g) parental goals for sleep. Demographic information (i.e., child age, diagnosis and ethnicity), information about the family context, and the child’s developmental history was also gathered at this time.
Sleep Measures
Parent-reported Sleep Diaries
Paper sleep diaries were completed by parents daily, across study phases. The diaries were used to calculate all dependent variables, and recorded information about the sleep setting; timing, frequency and duration (minutes) of daytime naps and NW; sleep onset latency (SOL; minutes); frequency of curtain calls (CC; i.e., bids for parental attention before sleep onset); the percentage of the night spent bed-sharing (minutes bed-sharing/total sleep duration × 100); and duration of early morning waking (EMW; minutes awake prior to 6am). Parents also recorded information about their child’s sleep interfering behavior and their response to the behavior.
Analysis of Video Footage
Swann Advanced-Series DVR4-1200 night-time recording hardware or TP-link Tapo C100 night vision Wi-fi cameras with micro-SD cards were provided to families with written operating instructions. Parents were instructed to turn the camera on when the child was put to bed and turn it off when the child arose in the morning. Video footage was primarily used to gather interobserver agreement data (IOA), however, in some cases it was used to complete sleep diaries that had missing information (one participant; Sally), and to inform the FBA process. Footage of the child’s sleep was obtained for a minimum of 30% of nights, across phases.
Sleep Problem Severity
Sleep Problem Severity (SPS) scores were calculated based upon parent-reported sleep diary data (9/10 participants), and data from video footage (1/10 participants), using the final seven nights of the baseline and treatment phases, and for the duration of short- (STFU) and long-term follow-up (LTFU). Scoring criteria were developed for toddlers (2–4 years 11 months), children (5–12 years 11 months) and adolescents (13–18 years) based on Richman et al. (1985) and National Sleep Foundation Guidelines (Hirshkowitz et al., 2015). For scoring criteria see Online Resource 1. McLay, France, Blampied, van Deurs et al. (2021) and McLay et al. (2019a, b) previously used this measure to evaluate sleep in autistic children. The total score for each of the seven nights was divided by seven to determine an average score for that period repeated across study phases. Based on the convention established by Richman et al. (1985) a score greater than two is indicative of a clinical level of sleep-problem severity.
Children’s Sleep Habits Questionnaire
The Children’s Sleep Habits Questionnaire (CSHQ; Owens et al., 2000) is a 45-item, parent-report measure used to assess a range of sleep difficulties in four-to-ten-year-old children. It yields a total sleep disturbance score and eight subscale scores: Bedtime resistance, Delayed SOL, Sleep duration, Sleep anxiety, Night waking, Sleep disordered breathing, Daytime sleepiness, and Parasomnias. Total scores greater than 41 indicate clinically significant sleep disturbance. The CSHQ is the most widely used standardized measure of sleep problems in children with NDD (e.g., Lambert et al., 2016; May et al., 2015; Moss et al., 2014).
There were two participants; Sally and Jimmy, who were outside of the age range for the CSHQ, however, the CSHQ was still administered to ensure consistency across participants. The CSHQ has been used extensively in populations outside the recommended age range, especially for children with NDD (Moss et al., 2014). There was no alternative parent-report measure that would allow for comparison across participants of varying ages (Owens et al., 2000).
Measures of Collateral Intervention Effects
The Child Behavior Checklist (CBCL), Depression, Anxiety and Stress Scale (DASS-21), Pittsburgh Sleep Quality Index (PSQI) and Relationship Quality Index (RQI) were completed by parents at pre- and post-treatment to assess collateral effects of sleep intervention on child emotional and behavioral problems, and parent sleep, relationship quality and wellbeing.
Child Behavior Checklist
The CBCL (Achenbach & Rescorla, 2001) is a parent-report measure of behavioral, social and emotional symptoms in children and adolescents. The CBCL for children between the ages of 1.5–5 years has seven subscales: Emotionally Reactive, Anxious/Depressed, Somatic Complaints, Withdrawn, Attention Problems, Aggressive Behavior, and Sleep Problems, and the version for children between the ages of six to 18 years has eight subscales: Aggressive Behavior, Attention Problems, Rules Breaking Behavior, Anxious/Depressed, Somatic Complaints, Social Problems, Thought Problems and Withdrawn/Depressed (Achenbach & Rescorla, 2001). Specific subscale scores are summed to provide internalizing and externalizing problem scores, and all subscale scores are summed to provide a total score. Higher scores indicate greater symptom severity (Normal < 60; Borderline 60–63; Clinical > 64).
Depression, Anxiety and Stress Scale
The DASS-21 (Henry & Crawford, 2005; Lovibond & Lovibond, 1995) is a self-report measure of symptoms of Depressive, Anxiety and Stress symptoms. Higher scores indicate greater symptom severity. Score ranges for classification of symptom severity for each subscale are indicated in Table 2.
Pittsburgh Sleep Quality Index
The PSQI (Buysse et al., 1989) is a self-report measure of sleep quality in adults. The PSQI has 19 items grouped into seven subscales: Sleep Quality, Sleep Latency, Sleep Duration, Sleep Efficiency, Sleep Disturbances, Sleep Medication Use and Daytime Sleepiness. Subscale scores are summed to provide a global sleep quality score; higher scores indicate poorer sleep quality, with scores greater than five reliably differentiating poor from good sleep quality.
Relationship Quality Index
The RQI (Norton, 1983) is a six-item, self-report measure of one’s perception of relationship quality with their partner. Item scores are summed to provide a total relationship quality score, higher scores indicating better relationship quality, with scores < 29 reliably differentiating poor from good relationship quality.
Treatment Acceptability
The Treatment Acceptability Rating Form – Revised (TARF-R; Reimers et al., 1992) is a parent-report measure of treatment satisfaction. It has 20 items grouped into six subscales: Reasonableness, Effectiveness, Cost, Willingness, Side-Effects and Disruption/Time. Seventeen items relate to ratings of treatment acceptability and three items assess problem severity and parents’ understanding of interventions. Ratings from the 17 acceptability items are summed to provide a total treatment acceptability score. Higher scores indicate greater acceptability.
Inter-Observer Agreement (IOA) and Treatment Fidelity
IOA data were calculated by comparing sleep diary and video recording data, in cases where sufficient video (> 30% of nights) was recorded (5/10 cases). IOA was only evaluated where behaviors were detectable by parents (i.e., quiet awakenings in which the child remained in their bed were excluded). Agreement for measures of time (e.g., duration of NWs, SOL) were noted if the video and sleep diary data were within 15 min. Percentage of agreement was calculated using the formula: [Agreement/(Agreement + Disagreement)] × 100. Treatment fidelity data was calculated by comparing the components of each child’s treatment protocol, with procedures noted in parent-reported diaries, video recordings, and daily contact notes. This was calculated for at least 30% of treatment and follow-up phases.
Procedures
Following clinical interviews, the following phases were undertaken: FBA and behavioral case formulation; baseline; treatment; STFU and LTFU.
Functional Behavioral Assessment (FBA) and Behavioral Case Formulation
An FBA was completed for each child using data from the clinical interview, SATT, parent-reported sleep diaries, and video footage. FBA outcomes determined the antecedent and consequence variables maintaining the sleep problem and enabled formulation of a hypothesis regarding the function(s) of sleep interfering behavior(s) for each child. Once all assessment data was obtained, behavioral case formulation and intervention planning occurred (BCFIP; Blampied, 2013; see Table 3 for FBA outcomes). The principle of less restriction was explicitly used in intervention planning to select and order the intervention strategies. Examples provided in intervention descriptions.
Baseline
Participants were randomly allocated a baseline length of 7, 14, or 21 days, though due to extraneous factors (e.g., child illness) or instability in baseline trends the baseline length was extended for two participants. During baseline, parents were instructed to continue with their typical routine and responses to their child’s behavior at bedtime and during the night.
Intervention
Sleep interventions commenced immediately upon conclusion of baseline. Interventions were limited to sleep/wake rescheduling, antecedent modifications, and reinforcement, though in one case (Sue), a modified extinction procedure was trialled briefly to resolve the sleep problem. In this case it was ultimately deemed inappropriate for the family, given high rates of seizure activity. These intervention components were implemented concurrently for 7/10 participants and sequentially for 3/10 participants (Rick, Heath and Rita). With sequential implementation, treatment components were ordered based on the less restrictive principle: circadian modifications, followed by antecedent modifications and then reinforcement (Kazdin, 2013; van Deurs et al., 2021). Interventions continued until the family’s treatment goals had been met, the sleep problem had resolved (as determined by sleep outcome data), or the participants withdrew from treatment.
Sleep/wake rescheduling was implemented for all but one participant (Sue), and for some participants included elimination of daytime naps (3/10 participants) and faded bedtime procedures (6/10 participants). Faded bedtime procedures involved delaying the child’s bedtime to the average time of sleep onset during baseline (Hirshkowitz et al., 2015). The bedtime was then bought forward in 15-min increments when the child consistently (typically after three–four consecutive nights) fell asleep within 15 min of being put to bed. This continued until the parents’ goal bedtime was met.
Antecedent modifications were required for all but one participant (Sally) who only required sleep/wake rescheduling. Antecedent modifications included changes to the sleep setting (2/10 participants), scheduled pre-bedtime access to digital devices (2/10 participants), establishing a consistent bedtime routine (3/10 participants), and the use of prompts and visual supports such as social stories (7/10 participants) and Gro-Clocks™ which provide a discriminative stimulus for sleep onset and offset (4/10 participants).
Reinforcement was provided for sleep-facilitative behavior for five participants. Treatment components for each participant are outlined in Table 3.
Maintenance and Follow-ups
Following intervention, participants entered the maintenance phase where researcher-initiated contact ceased, and data collection was discontinued. During this phase, parents completed post-treatment psychometrics and a post-treatment interview. STFU and LTFU data was collected for one week, 4–6 and 12–14-weeks post-treatment, respectively, for six/ten participants.
Data Analysis
Visual Analysis
Visual analysis was used to evaluate the effectiveness of interventions. Data from parent-reported sleep diaries, and from video (in cases which had missing diary data e.g., Sally) was graphed using SigmaPlot 14 software (systatsoftware.com) and the level, trend and variability of the data points were analysed across study phases. Percentage Below the Median (PBM; an effect size estimate; Parker et al., 2011) was calculated using the final week of intervention data for each participant (consistent with SPS calculations), across dependent variables. A PBM > 90% represents high treatment effectiveness; 70%–90% represents moderate effectiveness; and < 70% represents ineffective treatment (Ma, 2006, 2009). See Table of PBM results in Online Resource 2.
Modified Brinley Plots
Modified Brinley Plots are a form of scatterplot which display both idiographic and nomothetic treatment effect information, (i.e., individual participant data within the context of other individuals in the same group; Blampied, 2017) and were used to assess change in SPS and CSHQ scores across phases. Participants’ pre- and post-treatment scores are presented on the X and Y axis, respectively. Where each data point lies relative to the 45° diagonal line (i.e., where X equals Y) indicates the degree of change for each participant. If data points lie near or on the line, this means there was little or no change following intervention. Data points below the line indicate a decrease in scores, and vice versa (Blampied, 2017). Additional lines are displayed to provide information about where scores sit relative to the measure’s clinical cut-off (e.g., a score of 41 on the CSHQ).
Two effect size measures were calculated for SPS and CSHQ group data using Lakens (2013) software: The within-subjects Cohen’s dav (Cohen, 1988; Lakens, 2013) and the Percent Superiority Effect Size (PSES, also known as the Common Language Effect Size; McGraw & Wong, 1992). Cohen’s dav effect sizes were interpreted based on the criteria developed by Cohen (1988): Small effect dav ≤ 0.2, medium effect dav = ~ 0.5, and large effect dav ≥ 0.8. A reduction in SPS and CSHQ scores from pre- to post-treatment reflect change in a clinically desirable direction, represented by negative dav values. The PSES represents the probability (expressed as a percentage) that a randomly selected participant will have a post-treatment score clinically better than their pre-treatment score (Lakens, 2013; McGraw & Wong, 1992). Confidence intervals (CI) about dav (calculated using Cumming, 2012) were also calculated to classify dav as reliably different from zero (p < 0.05). Due to the small sample size, effect size calculations are based on autistic and RGND participants, combined.
Results
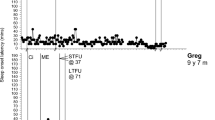
Data presented for each participant are those where the dependent variable reported was a treatment target. Two participants (Sue and Rick) had a large amount of data, due to a long intervention period. To ensure the graphs are readable, 85 and 61 data points were removed from mid-intervention for Sue and Rick respectively, and ten data points were removed from the start of baseline for Sue, these being periods of data stability. Graphs are grouped according to diagnostic group (autism or RGND) to facilitate comparison across NDD. Study phases, including major changes to treatment are depicted on each graph with phase change lines. Follow-up data is unavailable for three participants who withdrew during treatment or maintenance. SPS and CSHQ scores, collateral effects, IOA, TF, and treatment acceptability data follow below.
Treatment Outcomes
Excessive SOL
Excessive SOL was problematic (i.e., > 15 min) for seven participants (autism: Ritchie, Rita, Rick and Rob; RGND: Finn, Sally and Sue). Four of these participants showed an immediate reduction following intervention while two (Rita and Sue) showed an emergent treatment effect, and the other (Rick) showed minimal improvement. The average SOL across autistic participants ranged from 36–102 min at baseline and reduced to 8–40 min with intervention (see Fig. 1a). PBM values range from 86%–100% indicating moderate to large intervention effects. The average SOL across RGND participants ranged from 36–160 min at baseline and reduced to 22–37 min with intervention (see Fig. 1b). PBM values range from 71%–100% indicating moderate to large intervention effects. Improvements were maintained at follow-up (PBM = 100%). Overall, SOL was more delayed in children with RGND compared to the autistic children and less restrictive interventions were effective at reducing SOL. However, SOL remained higher, and above the ≤ 15-min clinical target, for RGND, suggesting lower treatment response compared to autism.
Frequency of CCs
CCs were problematic (i.e., > 1) for three autistic participants (Ritchie, Rob and Rita). All three of these participants exhibited improvements following intervention (see Figure in Online Resource 3). The frequency of CC’s ranged from 0 to 10 (M = 1.3–3, SD = 1.6–2.9) at baseline and reduced to zero to five (M = 0.17–1, SD = 0.45–1.13) with intervention. PBM values were 0%, 93% and 100% for Ritchie, Rob and Rita respectively, indicating no treatment effect for Ritchie but large treatment effects for Rob and Rita. Ritchie’s PBM results are due to a floor effect at baseline, with a median of zero, despite three baseline nights with between two and four CCs. One participant (Rita) showed an emergent treatment effect, with a consistent absence of CCs following phase three of intervention (sleep/wake rescheduling and a consistent bedtime routine). Improvements were not maintained at follow-up as Rob showed a slight increase in level (PBM of 43% of data below one CC).
Frequent Night Wakings (NWs)
Frequent NWs were problematic (i.e., > 0) for five participants (autism: Heath; RGND: Miri, Sue, Sally and Finn). Heath (autistic) exhibited an immediate reduction in the frequency of NWs following intervention (sleep/wake rescheduling and bedtime routine) from 0.4 at baseline to 0.1 with intervention (PBM = 90%: a large effect; see Fig. 2a).
Results for participants with RGND were mixed (PBM range = 14%–100%). The average frequency of NWs per participant with a RGND ranged from 1.1–3 at baseline and reduced to 0–0.9 with intervention (see Fig. 2b). Two (Finn and Sally) of the four participants exhibited a clear reduction in the frequency of NWs following intervention (PBM = 100%; a large effect). Treatment effects were maintained at follow-up. Miri also demonstrated improvements with intervention, however, the PBM value was low (50%), indicating ineffective treatment. This is likely due to a floor effect (baseline median = 1). Lastly, Sue exhibited no improvement in the frequency of NW (PBM = 14%). There was an initial increase in the frequency of NWs with changes to the antecedent stimuli for sleep (rocking in the hammock), however, data returned to baseline levels during treatment and increased above baseline levels at STFU (PBM = 0%). The frequency of NW reduced to baseline levels during LTFU (PBM = 14%). Overall, NWs were more frequent in children with RGND compared to the autistic children and intervention effects were variable.
Prolonged NWs
Prolonged NWs were problematic (i.e., > 5 min) for five participants (autism: Heath and Rick; RGND: Finn, Sally and Sue). All participants exhibited a reduction in NW duration following intervention. The average total duration of NWs per autistic participant ranged from 19–21 min at baseline and reduced to four minutes with intervention (see Fig. 3a). PBM values were 0% and 100% for Rick and Heath respectively, suggesting no intervention effect for Rick (due to a median = 0) but a large effect for Heath. Effects were maintained at follow-up for Heath (PBM = 100%). The average duration of NWs per participant with RGND ranged from 61–139 min at baseline and reduced to 0–36 min with intervention (PBM = 100%; a large intervention effect; see Fig. 3b). This was maintained at follow-up. Overall, less restrictive interventions resulted in a reduction in the duration of NWs for all participants. Prolonged NWs were more severe in children with RGND at baseline, but children in both groups showed the same response to treatment.
Duration of EMW
EMW was problematic (i.e., waking before 6am) for two autistic participants (Heath and Rick). Both participants exhibited improvements with intervention (baseline range 30–85 min; treatment range 11–14 min; see Figure in Online Resource 4) though with considerable variation for Rick for whom reductions in EMW occurred following sleep/wake rescheduling (phase one). PBM values were 57% and 100% for Rick and Heath respectively, indicating no effect for Rick but a large effect for Heath. For Heath, improvements were maintained at STFU (PBM = 100%) but an increase in EMW occurred at LTFU, although not to baseline levels (PBM = 100%). Overall, EMW was a greater problem for autistic participants with EMW rare (14%–15% of baseline nights) and minimal (< 30 min) in two participants with RGND (Miri and Finn).
Percentage of the Night Spent Bed-sharing
Bed-sharing was problematic for three participants with RGND (Miri, Jimmy and Sue). All participants exhibited an immediate reduction in bed-sharing following intervention (baseline range 35%–100%; treatment range 4%–18%; PBM = 82%–100%, a medium—large effect; see Figure in Online Resource 5). Improvements were maintained for most of the intervention phase, with the exception of Miri who resumed bed-sharing near the end of the intervention, and Sue who displayed variability in bed-sharing patterns across phases. Improvements were maintained at follow-up for Jimmy (PBM = 100%). Sue had a PBM of 0% at STFU due to illness, but a reduction in level at LTFU (PBM = 100%).
Sleep Problem Severity
Mean change in SPS scores across phases, is shown in Figs. 4a and b. At baseline SPS scores were variable, ranging from 3–8 and 4–11, for autistic and RGND participants respectively, confirming all were in the clinical range (> 2) prior to intervention. SPS scores reduced post-treatment for all participants (baseline: autism M = 5.2, SD = 1.9; treatment: autism M = 1.4, SD = 1.1; baseline RGND M = 8.4, SD = 2.9; treatment M = 3, SD = 1.9). Data were only available for 2/5 autistic participants and 3/5 participants with RGND at STFU and 3/5 participants for both groups at LTFU. For these participants, SPS scores remained below baseline levels, however, there was a very small deterioration in SPS scores between STFU and LTFU. Overall, the baseline mean SPS score of 6.8 (SD = 2.9) reduced to 2.2 (SD = 1.7), 3.1 (SD = 3.6) and 2.3 (SD = 2.2) at post-treatment, STFU and LTFU respectively.
Figure 4a and b also display the Cohen’s dav effect size, 95% CI and PSES for SPS for combined autism and RGND groups. From baseline to post-treatment the effect is large (dav = -1.96; 95% CI = -3.1, -0.8; PSES = 98%). This was maintained at STFU (dav = -1.08; 95% CI = -1.9, -0.2; PSES = 92%) and LTFU (dav = -1.59; 95% CI = -2.8, -0.4; PSES = 96%).
Children’s Sleep Habits Questionnaire
Change in CSHQ scores from baseline to post-treatment is shown in Online Resource 6. Seven out of ten parents of participants (5/5 RGND; 2/5 autism) completed the CSHQ at pre- and post-treatment. At baseline CSHQ scores were variable (autism range 41–58; RGND range 47–71) though all were in the clinical range (> 41). These scores reduced post-treatment for all but one participant (Miri). Mean CSHQ scores reduced post-treatment for autistic and RGND groups (baseline: autism M = 51.4, SD = 6.47; treatment: autism M = 40, SD = 2.0; baseline RGND M = 56.6, SD = 8.59; treatment RGND M = 47.2, SD = 6.31). These post-treatment scores were below the clinical cut-off for autism only. Figure S4 also displays the Cohen’s dav effect size, 95% CI and PSES for the CSHQ scores (dav = -1.33; 95% CI = -2.3, -0.24; PSES = 90%) demonstrating a large treatment effect.
Collateral Effects of Intervention
A summary of results from psychometrics measuring collateral effects are outlined below. For specific psychometric results across participants refer to Table 2.
Child Behavior Checklist
Eight parents completed the CBCL at pre- and post-treatment (four autism; four RGND). For RGND participants, mean changes in internalizing, externalizing and total CBCL scores were negligible, with an increase in internalizing problems (they remained in the borderline range), reduction in externalizing problems and no change for total scores (both remained in normal range). By contrast, mean scores reduced across all domains for participants in the autistic group. Mean CBCL total scores reduced from the clinical to borderline range, internalizing problem score from the borderline to normal range, and externalizing scores remained in the clinical range. Mean total CBCL scores were higher for autistic participants compared to RGND at both pre- and post-treatment, suggesting autistic children had greater behavioral, emotional and social difficulties, and greater collateral effects.
Parent Depression, Anxiety and Stress
Nine mothers (four autism; five RGND) and six fathers (two autism; four RGND) completed the DASS-21 at pre- and post-treatment. There was a major floor effect for the DASS-21, with scores consistently low (in the normal range) during baseline for most parents. Post-treatment DASS-21 scores for all parents mostly remained the same or similar to pre-treatment scores and estimate normal category levels of symptoms. However, a considerable elevation is evident in the Stress scale for fathers of children with RGND (M = 2.8 to 7.0), suggesting paternal stress levels increased following intervention, however, scores remained within the normal range. On average, parents of autistic children had higher depression, anxiety, and stress at both pre- and post-treatment compared to parents of children with RGND.
Parent Sleep Quality
Two and five sets of parents (mothers and father) of autistic and RGND participants respectively, completed the PSQI pre- and post-treatment. For parents of children with RGND, mean maternal scores were similar at pre- and post-treatment and remained above the clinical cut-off, suggesting sleep remained poor. All paternal scores increased from below cut-off (M = 4.5) at pre-treatment to above cut-off (M = 6.5) post-treatment, suggesting a deterioration in paternal sleep quality. In comparison, PSQI scores of parents of autistic children reflect improvements in parental sleep quality following intervention. All maternal PSQI scores reduced from above cut-off at pre-treatment (M = 10.5) to below cut-off at post-treatment (M = 4.3). Although to a lesser extent, mean paternal PSQI scores also reduced following child sleep intervention. Overall, parental sleep quality was generally poorer for parents of autistic children compared to RGND during baseline, and differences between groups could be due to a floor effect for RGND parents.
Parent Relationship Quality
Two and three sets of parents of autistic and RGND participants respectively, completed the RQI at pre- and post-treatment. There was a ceiling effect on the RQI for parents of children with RGND, with all scores estimating high levels of relationship quality pre-treatment. No notable changes in total RQI scores were evident post-treatment, with most remaining the same. Results were mixed for parents of autistic children. Mean maternal scores reduced following intervention, suggesting a deterioration in relationship satisfaction and their scores remained below the cut-off estimating poor relationship quality, but mean paternal scores increased, remaining above the cut-off estimating good relationship quality. Overall, relationship satisfaction was generally lower for parents of autistic children compared to RGND.
Treatment Acceptability
Parents of 8/10 children (seven mothers and two fathers) completed the TARF-R. Subscale and total scores are displayed in Table in Online Resource 7. Total acceptability scores ranged from 93.5 to 119. Mean total TARF-R scores are 102.1 and 107.5 for RGND and autistic groups respectively. Average ratings of parents of children with RGND yielded higher scores in the Willingness (RGND = 19.6; autism = 18.5) subscale, and lower scores in the Reasonableness (RGND = 18.9; autism = 20.3), Side Effects (RGND = 15.8; autism = 19.5) and Disruption (RGND = 15.7; autism = 18) subscales, when compared to autism. In spite of some variation, parents’ ratings yielded scores which suggest they perceived interventions to be acceptable, understandable, and effective.
Interobserver Agreement (IOA)
IOA data were able to be calculated for 5/10 participants but not for the rest due to problems with video recording and/or quality (4/5 participants), and lack of sleep diary information (i.e., dependent variables not detectable to parents; 1/5 participants). Mean IOA was 86% (range, 80%–92%) across all study phases and behaviors.
Treatment Fidelity
Treatment fidelity was calculated for 30%–100% of nights across phases. Mean treatment fidelity was 74% (range 29%–100%). For 7/10 participants treatment fidelity scores were similar across study phases but for 2/10 participants (Heath and Rob) treatment fidelity increased and 1/10 (Sue) decreased from treatment to follow-up. Mean treatment fidelity was 72% and 79% at STFU and LTFU respectively. Treatment fidelity varied across diagnostic groups, with a mean treatment fidelity of 87% and 53% for autism and RGND respectively. Treatment fidelity was low (e.g., 29% and 57%) for two participants with RGND (Miri and Sue). Mean treatment fidelity for RGND excluding their scores was 68%.
Discussion
This study evaluated the effectiveness and acceptability of behavioral interventions explicitly planned based on the principle of less restriction for sleep problems in autistic children and children with RGND. The collateral effects of these interventions on children’s daytime behavior, and parental wellbeing, sleep, and relationship quality were also evaluated. Results indicated that less restrictive behavioral interventions were effective in reducing sleep problems across all NDD, although collateral effects varied.
As reflected in prior research, problems with initiating and maintaining sleep were the most identified difficulty (Krakowiak et al., 2008; Richdale & Schreck, 2009; Woodford et al., 2021). Across children, sleep problems were underpinned by a combination of behavioral factors and circadian dysregulation and were frequently underpinned by an absence of appropriate discriminative stimuli for sleep, e.g., variation in sleep setting and inconsistent routines. Consequences (i.e., contingencies of reinforcement) that consistently played a role in the maintenance of sleep problems were parental attention, access to tangible items such as devices, toys and food, and in one case, avoidance of bed. Insufficient sleep pressure, both a precursor and product of circadian dysregulation, was also a common precipitating factor to sleep disturbance across participants.
Interventions used in this study and explicitly selected using the less restrictive alternative principle, included a combination of sleep/wake rescheduling strategies, sleep hygiene modifications, visual supports, and rewards. These interventions were effective in reducing sleep problems across all participants, including SOL, CCs, NWs, bed-sharing and EMW. Although the frequency of NWs did not fully resolve in those with RGND, the duration reduced considerably, as did bed-sharing, suggesting that children with RGND learned to settle independently and expeditiously, following a NW. Improvements remained at follow-up for six/seven participants. Nine participants (90%) required some form of circadian modification (e.g., elimination of daytime naps, sleep/wake rescheduling), with circadian modification alone effective in reducing sleep problems such as NW in two participants (Sally and Rick). It is likely circadian modifications altered motivation for sleep (i.e., increased the reinforcement value of sleep) through an increase in homeostatic sleep pressure (Laraway et al., 2003; Piazza et al., 1991, 1997). Furthermore, modifications of sleep hygiene are likely to have improved sleep by creating consistent discriminative stimuli for sleep onset and offset, as well as a sleep-facilitative bedtime routine and bedroom environment (Blampied & Bootzin, 2013).
Prior research has demonstrated the effectiveness of behavioral interventions for sleep, but typically including extinction or modified extinction procedures (Piazza et al., 1991, 1997; van Deurs et al., 2019). Our results suggest that less restrictive interventions, as defined in this study, may be an effective alternative to extinction-based procedures, even among behaviors that appear to be maintained by social attention and access to tangible items. These findings are consistent with limited research that has demonstrated the effects of antecedent modifications including circadian manipulations in treating sleep disturbances in autistic children (Christodulu & Durand, 2004; van Deurs et al., 2019, 2021) and children with RGND (Piazza et al., 1991, 1997).
The preliminary comparisons possible in this study suggest that there were differences in sleep problem type and topography between RGND and autism groups. Generally, those with RGND had greater problems with SOL, NWs and bed-sharing at baseline, while those with autism had greater problems with CCs and EMW. The determined function(s) of the sleep problems also varied, with access to devices more common among autistic children and social attention among those with RGND. Further, overall sleep problem severity was greater in children with RGND, possibly reflecting the multitude of causal factors that may underpin sleep problems in this group (Stores, 2016). The extent to which these differences reliably characterise autistic children versus those with RGND remains to be determined by further research.
Research has reported that parents of autistic children perceive behavioral sleep interventions to be acceptable (McLay et al., 2017; McLay, France, Blampied, van Deurs et al., 2021; McLay et al., 2019a, b; van Deurs et al., 2021), however, research is limited with children with RGND (Allen et al., 2013). This study demonstrates that parents of autistic children and children with RGND find parent-implemented less restrictive behavioral interventions to be acceptable. Parents of children with RGND found the interventions to be more disruptive and to have more side effects when compared to those parenting autistic children. While the small sample limits the robustness of these findings, it is possible that they reflect the complexity of managing sleep problems in children with RGND and the correspondingly increased parental burden associated with implementing a behavioral sleep intervention (Beresford et al., 2016; Priday et al., 2017).
The collateral effects of less restrictive interventions on child behavior, and parent relationship quality, well-being and sleep were mixed (Hunter et al., 2020; Malow et al., 2014; McLay et al., 2021a, b, c). Results suggest that sleep intervention may have a positive effect (i.e., reduction) on internalizing and externalizing symptoms in autistic children, a finding consistent with that of existing research (Hunter et al., 2020; McLay et al., 2021a, b, c). However, changes in internalizing and externalizing symptoms were mixed in children with RGND. This difference across groups could be associated with the additional comorbidities within children with RGND, many of which may not be directly influenced by sleep (e.g., medical, biological, and physical issues), although further replication is required to establish the reliability of these observations (Hunter et al., 2020; McLay et al., 2021a, b, c).
Although a small sample size limits the inferences that can be made, findings suggest that parent relationship quality, and depressive, anxiety and stress symptoms were largely unchanged following intervention, likely due to ceiling and floor effects respectively. The exception to this was stress which worsened over treatment among fathers of children with RGND, as did sleep quality. It is possible that this reflects increased involvement of fathers in sleep intervention, as a result of this study. However, improvements in sleep were evident in both mothers and fathers of autistic children.
Clinical Implications
These findings have several important clinical implications. Firstly, the data indicate a wide range of factors, including precursors to sleep problems (e.g., environmental, circadian, medical and intellectual factors), in addition to sleep interfering behavior (e.g., device use, attention seeking), need to be considered in the assessment and treatment of sleep problems in children with NDD (McLay, France, Blampied, van Deurs et al., 2021). Secondly, results suggest that the strategies implemented in this study may be minimally sufficient for the treatment of sleep disturbance and confirm that there are effective less restrictive alternatives to extinction-based procedures. Lastly, data suggests that there is potential for behavioral sleep intervention, to address a range of co-occurring behaviors characteristic of children with NDD, as well as parent-related variables (e.g., sleep in parents of autistic children) (Stores, 2016). With parents of children with NDD experiencing increased stress, likely exacerbated by challenging child behavior and vice versa, such findings highlight the importance of considering these variables in treatment (Hunter et al., 2020).
Limitations
There are several limitations to this study which should be considered. Firstly, treatment fidelity was low, specifically in those with RGND. Low treatment fidelity can not only negatively impact treatment outcomes but reduces the ability to attribute outcomes to the interventions and opportunities for replication of interventions in future studies (Miller & Rollnick, 2014). However, there was an obvious difference in treatment response in those families who adhered to the treatment programme and those who did not. For example, there was a lapse in treatment adherence near the end of intervention for Miri, which resulted in deterioration in bed-sharing and NW. Second, this study evaluated the effectiveness of individualized and multi-component interventions in most cases, which precludes evaluation of the effectiveness of individual treatment components. Third, interventions were time intensive and required considerable clinical support, which is not always readily available in clinical settings. Fourth, a detailed understanding of the child’s health status, how it fluctuated throughout intervention and the impact on treatment response was lacking. Finally, the number of replications was small and small sample sizes precluded formal quantitative comparison across groups. The degree to which the children and families studied here are representative of children and families with NDD is unknown, and therefore, the generality of the findings can only be determined by direct, systematic, and clinical replication.
Recommendations for Future Research
Future research should separate treatment components further so that circadian and antecedent modifications are staggered, enabling researchers to determine what components are necessary and sufficient for positive change. In addition, research should consider the degree to which medical, biological and intellectual factors predict and moderate children’s response to behavioral sleep interventions, through quantification of complexity (both of child presentation and family structure and circumstances). Evaluation of the relationship between these factors and treatment process and outcome would help enhance generalizability. Furthermore, research is needed to determine whether sleep intervention has collateral effects on a range of child and parent variables (e.g., child behavior, parent well-being), specifically in children with RGND. The impact on medical and biological factors (e.g., health-related quality of life, the circadian rhythm of melatonin; Woodford et al., 2021) should also be assessed, to further evaluate the interface between the biology and behavioral context of sleep. Finally, future research with direct, systematic, and clinical replication is needed to establish best practice with respect to sleep interventions for children with NDD (McLay et al., 2021; Woodford et al., 2021).
References
Achenbach, T. M., & Rescorla, L. A. (2001). Manual for the ASEBA school-age forms & profiles: Child behavior checklist for ages 6–18, teacher's report form, youth self-report: An integrated system of multi-informant assessment. University of Vermont, Research Center for Children Youth & Families.
Ainsworth, M. D. (1969). Object relations, dependency, and attachment: A theoretical review of the infant-mother relationship. Child Development, 40(4), 969–1025. https://doi.org/10.1111/j.1467-8624.1969.tb04561.x
Alabaf, S., Gillberg, C., Lundström, S., Lichtenstein, P., Kerekes, N., Råstam, M., & Anckarsäter, H. (2019). Physical health in children with neurodevelopmental disorders. Journal of Autism and Developmental Disorders, 49(1), 83–95. https://doi.org/10.1007/s10803-018-3697-4
Allen, K. D., Kuhn, B. R., DeHaai, K. A., & Wallace, D. P. (2013). Evaluation of a behavioral treatment package to reduce sleep problems in children with Angelman Syndrome. Research in Developmental Disabilities, 34(1), 676–686.
Angriman, M., Caravale, B., Novelli, L., Ferri, R., & Bruni, O. (2015). Sleep in children with neurodevelopmental disabilities. Neuropediatrics, 46(3), 199–210.
Barlow, D. H., Nock, M., & Hersen, M. (2009). Single case experimental designs: Strategies for studying behavior for change (3rd ed.). Pearson Education.
Beresford, B., Stuttard, L., Clarke, S., & Maddison, J. (2016). Parents’ experiences of psychoeducational sleep management nterventions: A qualitative study of parents of children with neurodevelopmental disabilities. Clinical Practice in Pediatric Psychology, 4(2), 164–175. https://doi.org/10.1037/cpp0000144
Blampied, N. M. (2013). Functional behavioral analysis of sleep in infants and children. In A. R. Wolfson & H. Montgomery-Downs (Eds.), The Oxford handbook of infant, child, and adolescent sleep and behavior (pp. 169–188). Oxford University Press.
Blampied, N. M. (2017). Analysing therapeutic change using modified Brinley plots: History, construction, and interpretation. Behavior Therapy, 48(1), 115–127. https://doi.org/10.1016/j.beth.2016.09.002
Blampied, N. M., & Bootzin, R. R. (2013). Sleep: A behavioral account. In G. J. Madden, W. V. Dube, T. D. Hackenberg, G. P. Hanley, & K. A. Lattal (Eds.), APA handbook of behavior analysis: Vol. 2. Translating principles into practice (pp. 425–453). American Psychological Association. https://doi.org/10.1037/13938-017
Blampied, N. M., & France, K. G. (1993). A behavioral model of infant sleep disturbance. Journal of Applied Behavior Analysis, 26(4), 477–492.
Blunden, S., Etherton, H., & Hauck, Y. (2016). Resistance to cry intensive sleep intervention in young children: Are we ignoring children’s cries or parental concerns? Children, 3(2), 8. https://doi.org/10.3390/children3020008
Buysse, D. J., Reynolds, C. F., III., Monk, T. H., Berman, S. R., & Kupfer, D. J. (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213.
Carnett, A., Hansen, S., McLay, L., Neely, L., & Lang, R. (2020). Quantitative-analysis of behavioral interventions to treat sleep problems in children with autism. Developmental Neurorehabilitation, 23(5), 271–284. https://doi.org/10.1080/17518423.2019.1646340
Christ, T. J. (2007). Experimental control and threats to internal validity of concurrent and nonconcurrent multiple baseline designs. Psychology in the Schools, 44(5), 451–459. https://doi.org/10.1002/pits.20237
Christodulu, K. V., & Durand, V. M. (2004). Reducing bedtime disturbance and night waking using positive bedtime routines and sleep restriction. Focus on Autism and Other Developmental Disabilities, 19(3), 130–139. https://doi.org/10.1177/10883576040190030101
Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Lawrence Earlbaum Associates. https://doi.org/10.4324/9780203771587
Cumming, G. (2012). Understanding the new statistics: Effect sizes, confidence intervals, and meta-analysis. Routledge. https://doi.org/10.4324/9780203807002
Davis, A. S., Hoover, K. L., & Mion, A. M. (2018). Understanding and treating children and adolescents with neurodevelopmental disorders. In J. N. Butcher & P. C. Kendall (Eds.), APA handbook of psychopathology: Child and adolescent psychopathology (pp. 279–315). American Psychological Association.
Didden, R., Curfs, L. M., Sikkema, S. P., & de Moor, J. (1998). Functional assessment and treatment of sleeping problems with developmentally disabled children: Six case studies. Journal of Behavior Therapy and Experimental Psychiatry, 29(1), 85–97.
Etherton, H., Blunden, S., & Hauck, Y. (2016). Discussion of extinction-based behavioral sleep interventions for young children and reasons why parents may find them difficult. Journal of Clinical Sleep Medicine, 12(11), 1535–1543.
European Commission. (2021). Rare diseases: Commission activities in the area of rare diseases. http://ec.europa.eu/research/health/index.cfm?pg=area&areaname=rare
France, K. G., & Blampied, N. M. (1999). Infant sleep disturbance: Description of a problem behaviour process. Sleep Medicine Reviews, 3, 265–280.
France, K. G., & Blampied, N. M. (2005). Modifications of systematic ignoring in the management of infant sleep disturbance: Efficacy and infant distress. Child Family Behavior Therapy, 27(1), 1–16.
France, K. G., Henderson, J. M. T., & Hudson, S. M. (1996). Fact, act, and tact: A three-stage approach to treating the sleep problems of infants and young children. Child and Adolescent Psychiatric Clinics of North America, 5(3), 581–600. https://doi.org/10.1016/S1056-4993(18)30350-X
Ghanizadeh, A., & Faghih, M. (2011). The impact of general medical condition on sleep in children with mental retardation. Sleep and Breathing, 15(1), 57–62. https://doi.org/10.1007/s11325-009-0312-0
Goldman, S. E., Richdale, A. L., Richdale, A. L., Clemons, T., & Malow, B. A. (2012). Parental sleep concerns in autism spectrum disorders: Variations from childhood to adolescence. Journal of Autism and Developmental Disorders, 42(4), 531–538. https://doi.org/10.1007/s10803-011-1270-5
Hanley, G. P. (2005). Sleep assessment and treatment tool [Measurement instrument]. https://practicalfunctionalassessment.files.wordpress.com/2015/06/satt.pdf
Henry, J. D., & Crawford, J. R. (2005). The short-form version of the Depression Anxiety Stress Scales (DASS-21): Construct validity and normative data in a large non-clinical sample. British Journal of Clinical Psychology, 44(2), 227–239. https://doi.org/10.1348/014466505X29657
Hirshkowitz, M., Whiton, K., Albert, S. M., Alessi, C., Bruni, O., DonCarlos, L., Hazen, N., Herman, J., Katz, E. S., & Kheirandish-Gozal, L. (2015). National Sleep Foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health, 1(1), 40–43.
Hunter, J. E., McLay, L. K., France, K. G., & Blampied, N. M. (2020). Systematic review of the collateral effects of behavioral sleep interventions in children and adolescents with autism spectrum disorder. Research in Autism Spectrum Disorders, 79. https://doi.org/10.1016/j.rasd.2020.101677
Jin, C. S., Hanley, G. P., & Beaulieu, L. (2013). An individualized and comprehensive approach to treating sleep problems in young children. Journal of Applied Behavior Analysis, 46(1), 161–180. https://doi.org/10.1002/jaba.16
Johnston, J. M., & Sherman, R. A. (1993). Applying the least restrictive alternative principle to treatment decisions: A legal and behavioral analysis. The Behavior Analyst, 16(1), 103–115. https://doi.org/10.1007/BF03392615
Kazdin, A. E. (2013). Behavior modification in applied settings (7th ed.). Waveland Press.
Konjarski, M., Murray, G., Lee, V. V., & Jackson, M. L. (2018). Reciprocal relationships between daily sleep and mood: A systematic review of naturalistic prospective studies. Sleep Medicine Reviews, 42, 47–58. https://doi.org/10.1016/j.smrv.2018.05.005
Krakowiak, P., Goodlin-Jones, B., Hertz-Picciotto, I., Croen, L. A., & Hansen, R. L. (2008). Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: A population-based study. Journal of Sleep Research, 17(2), 197–206.
Kronk, R., Bishop, E. E., Raspa, M., Bickel, J. O., Mandel, D. A., & Bailey, D. B. (2010). Prevalence, nature, and correlates of sleep problems among children with fragile X syndrome based on a large scale parent survey. Sleep, 33(5), 679–687.
Kuhn, B. R., LaBrot, Z. C., Ford, R., & Roane, B. M. (2020). Promoting independent sleep onset in young children: Examination of the Excuse me drill. Behavioral Sleep Medicine, 18(6), 730–745. https://doi.org/10.1080/15402002.2019.1674852
Lakens, D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Frontiers in Psychology, 4, 1–12. https://doi.org/10.3389/fpsyg.2013.00863
Lambert, A., Tessier, S., Rochette, A.-C., Scherzer, P., Mottron, L., & Godbout, R. (2016). Poor sleep affects daytime functioning in typically developing and autistic children not complaining of sleep problems: A questionnaire-based and polysomnographic study. Research in Autism Spectrum Disorders, 23, 94–106.
Laraway, S., Snycerski, S., Michael, J., & Poling, A. (2003). Motivating operations and terms to describe them: Some further refinements. Journal of Applied Behavior Analysis, 36(3), 407–414.
Lovibond, P. F., & Lovibond, S. H. (1995). The structure of negative emotional states: Comparison of the depression anxiety stress scales (DASS) with the Beck depression and anxiety Inventories. Behaviour Research and Therapy, 33(3), 335–343.
Ma, H. H. (2006). An alternative method for quantitative synthesis of single-subject researches: Percentage of data points exceeding the median. Behavior Modification, 30(5), 598–617. https://doi.org/10.1177/0145445504272974
Ma, H. H. (2009). The effectiveness of intervention on the behavior of individuals with autism: A meta-analysis using percentage of data points exceeding the median of baseline phase (PEM). Behavior Modification, 33(3), 339–359. https://doi.org/10.1177/0145445509333173
Malow, B. A., Adkins, K. W., Reynolds, A., Weiss, S. K., Loh, A., Fawkes, D., Katz, T., Goldman, S. E., Madduri, N., Hundley, R., & Clemons, T. (2014). Parent-based sleep education for children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 44(1), 216–228. https://doi.org/10.1007/s10803-013-1866-z
May, T., Cornish, K., Conduit, R., Rajaratnam, S. M., & Rinehart, N. J. (2015). Sleep in high-functioning children with autism: Longitudinal developmental change and associations with behavior problems. Behavioral Sleep Medicine, 13(1), 2–18.
McGraw, K. O., & Wong, S. P. (1992). A common language effect size statistic. Psychological Bulletin, 111(2), 361–365. https://doi.org/10.1037/0033-2909.111.2.361
McLay, L., France, K., Blampied, N., Danna, K., & Hunter, J. (2017). Using functional behavioral assessment to develop a multicomponent treatment for sleep problems in a 3-year-old boy with autism. Clinical Case Studies, 16(3), 254–270.
McLay, L. K., France, K. G., Knight, J., Blampied, N. M., & Hastie, B. (2019a). The effectiveness of function-based interventions to treat sleep problems, including unwanted co-sleeping, in children with autism. Behavioral Interventions, 34(1), 30–51. https://doi.org/10.1002/bin.1651
McLay, L., Roche, L., France, K. G., Blampied, N. M., Lang, R., France, M., & Busch, C. (2019b). Systematic review of the effectiveness of behaviorally-based interventions for sleep problems in people with rare genetic neurodevelopmental disorders. Sleep Medicine Reviews, 46, 54–63.
McLay, L. K., France, K. G., Blampied, N. M., Hunter, J. E., van Deurs, J. R., Woodford, E. C., Gibbs, R., & Lang, R. (2021a). Collateral child and parent effects of function-based behavioral interventions for sleep problems in children and adolescents with autism. Journal of Autism and Developmental Disorders. https://doi.org/10.1007/s10803-021-05116-3
McLay, L., France, K., Blampied, N., van Deurs, J., Hunter, J., Knight, J., Hastie, B., Carnett, A., Woodford, E., Gibbs, R., & Lang, R. (2021b). Function-based behavioral interventions for sleep problems in children and adolescents with autism: Summary of 41 clinical cases. Journal of Autism and Developmental Disorders, 51(2), 418–432. https://doi.org/10.1007/s10803-020-04548-7
McLay, L. K., Schluter, P. J., Eggleston, M. J. F., Woodford, E. C., & Bowden, N. (2021c). Melatonin dispensing among New Zealand children aged 0–18 years with autism: A nationwide cross-sectional study. Sleep Medicine, 80, 184–192. https://doi.org/10.1016/j.sleep.2021.01.028
Middlemiss, W., & Kendall-Tackett, K. A. (2014). The science of mother-infant sleep: Current findings on bedsharing, breastfeeding, sleep training and normal infant sleep. Praeclarus Press.
Miller, W. R., & Rollnick, S. (2014). The effectiveness and ineffectiveness of complex behavioral interventions: Impact of treatment fidelity. Contemporary Clinical Trials, 37(2), 234–241. https://doi.org/10.1016/j.cct.2014.01.005
Mörelius, E., & Hemmingsson, H. (2014). Parents of children with physical disabilities—Perceived health in parents related to the child's sleep problems and need for attention at night. Child: Care, Health & Development, 40(3), 412–418. https://doi.org/10.1111/cch.12079
Moss, A. H. B., Gordon, J. E., & O’Connell, A. (2014). Impact of sleepwise: An intervention for youth with developmental disabilities and sleep disturbance. Journal of Autism and Developmental Disorders, 44(7), 1695–1707. https://doi.org/10.1007/s10803-014-2040-y
Norton, R. (1983). Measuring marital quality: A critical look at the dependent variable. Journal of Marriage and Family, 45(1), 141–151. https://doi.org/10.2307/351302
Odom, S. L., Horner, R. H., Snell, M. E., & Blacher, J. (2007). The construct of developmental disabilities. In S. L. Odom, R. H. Horner, M. E. Snell, & J. Blacher (Eds.), Handbook of developmental disabilities (pp. 3–14). The Guilford Press.
Owens, J. A., Spirito, A., & McGuinn, M. (2000). The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep, 23(8), 1043–1049. https://doi.org/10.1093/sleep/23.8.1d
Parish, J. M. (2009). Sleep-related problems in common medical conditions. Chest, 135(2), 563.
Parker, R. I., Vannest, K. J., & Davis, J. L. (2011). Effect size in single-case research: A review of nine nonoverlap techniques. Behavior Modification, 35, 303–322.
Piazza, C. C., Fisher, W., & Moser, H. (1991). Behavioral treatment of sleep dysfunction in patients with the Rett syndrome. Brain & Development, 13(4), 232–237.
Piazza, C. C., Fisher, W. W., & Sherer, M. (1997). Treatment of multiple sleep problems in children with developmental disabilities: Faded bedtime with response cost versus bedtime scheduling. Developmental Medicine and Child Neurology, 39(6), 414–418.
Priday, L. J., Byrne, C., & Totsika, V. (2017). Behavioural interventions for sleep problems in people with an intellectual disability: A systematic review and meta-analysis of single case and group studies. Journal of Intellectual Disability Research, 61(1), 1–15. https://doi.org/10.1111/jir.12265
Reimers, T. M., Wacker, D. P., Cooper, L. J., & DeRaad, A. O. (1992). Clinical evaluation of the variables associated with treatment acceptability and their relation to compliance. Behavioral Disorders, 18(1), 67–76.
Richdale, A. L., & Schreck, K. A. (2009). Sleep problems in autism spectrum disorders: Prevalence, nature, & possible biopsychosocial aetiologies. Sleep Medicine Reviews, 13(6), 403–411.
Richman, N., Douglas, J., Hunt, H., Lansdown, R., & Levere, R. (1985). Behavioural methods in the treatment of sleep disorders—A pilot study. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 26(4), 581–590.
Rigney, G., Ali, N. S., Corkum, P. V., Brown, C. A., Constantin, E., Godbout, R., Hanlon-Dearman, A., Ipsiroglu, O., Reid, G. J., Shea, S., Smith, I. M., Van der Loos, H. F. M., & Weiss, S. K. (2018). A systematic review to explore the feasibility of a behavioural sleep intervention for insomnia in children with neurodevelopmental disorders: A transdiagnostic approach. Sleep Medicine Reviews, 41, 244–254. https://doi.org/10.1016/j.smrv.2018.03.008
Roberds-Roach, D. L., Short, M. B., & Lerman, D. C. (2012). An intervention using graduated extinction to decrease bed-sharing in 2- to 6-year-old children. Child & Family Behavior Therapy, 34(2), 156–162. https://doi.org/10.1080/07317107.2012.684654
Sanberg, S. A., Kuhn, B. R., & Kennedy, A. E. (2018). Outcomes of a behavioral intervention for sleep disturbances in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 48(12), 4250–4277. https://doi.org/10.1007/s10803-018-3644-4
Shochat, T., Cohen-Zion, M., & Tzischinsky, O. (2014). Functional consequences of inadequate sleep in adolescents: A systematic review. Sleep Medicine Reviews, 18(1), 75–87. https://doi.org/10.1016/j.smrv.2013.03.005
Sparrow, S. S., Cicchetti, D. V., & Saulnier, C. A. (2016). Vineland adaptive behavior scales, third edition (Vineland-3). Pearson.
Stores, G. (2016). Multifactorial influences, including comorbidities, contributing to sleep disturbance in children with a neurodevelopmental disorder. CNS Neuroscience & Therapeutics, 22(11), 875–879. https://doi.org/10.1111/cns.12574
Thambirajah, M. S. (2011). Developmental assessment of the school-aged child with developmental disabilities: A clinician's guide. Jessica Kingsley Publishers.
van Deurs, J. R., McLay, L. K., France, K. G., & Blampied, N. M. (2021). Sequential implementation of functional behavior assessment-informed treatment components for sleep disturbance in autism: A case study. Behavioral Sleep Medicine, 19(3), 333–351. https://doi.org/10.1080/15402002.2020.1758701
van Deurs, J. R., McLay, L. K., France, K. G., Blampied, N. M., Lang, R. B., & Hunter, J. E. (2019). Behavioral sleep intervention for adolescents with autism spectrum disorder: A pilot study. Advances in Neurodevelopmental Disorders, 3(4), 397–410. https://doi.org/10.1007/s41252-019-00123-z
Volkmar, F. R., Rogers, S. J., Paul, R., & Pelphrey, K. A. (2014). Handbook of autism and pervasive developmental disorders (4th ed.). John Wiley & Sons.
Weiskop, S., Richdale, A., & Matthews, J. (2005). Behavioural treatment to reduce sleep problems in children with autism or fragile X syndrome. Developmental Medicine and Child Neurology, 47(2), 94–104.
Whittall, H., Kahn, M., Pillion, M., & Gradisar, M. (2019). Graduated extinction and its barriers for infant sleep problems: An investigation into the experiences of parents. Sleep Medicine, 64. https://doi.org/10.1016/j.sleep.2019.11.1163
Woodford, E. C., McLay, L., France, K. G., Blampied, N. M., Gibbs, R., Swan, C. E., & Eggleston, M. (2021). Endogenous melatonin and sleep in individuals with rare genetic neurodevelopmental disorders (RGND): A systematic review. Sleep Medicine Reviews, 57, Article 101433. https://doi.org/10.1016/j.smrv.2021.101433
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This research was supported by funding from the Royal Society of New Zealand Marsden Fund and Health Research Council of New Zealand.
Author information
Authors and Affiliations
Contributions
Emma C. Woodford: Conceptualization, Methodology, Formal analysis, Writing—original draft, Writing—review & editing, Visualization. Laurie K. McLay: Conceptualization, Methodology, Validation, Writing—review & editing, Supervision. Karyn G. France: Conceptualization, Validation, Writing—review & editing, Supervision. Neville M. Blampied: Conceptualization, Formal analysis, Writing—review & editing, Supervision. Rosina Gibbs: Formal analysis. Charis Whitaker: Writing – review and editing. Emma McCaughan: Formal analysis.
Corresponding author
Ethics declarations
Ethics Approval
This research was approved by the relevant university Human Ethics Committee (HEC 2018–48 and HEC 218–47) and has been conducted in accordance with the 1964 Helsinki declaration and its later amendments.
Informed Consent
Informed consent was obtained from all parent participants and, consent or assent was provided by most children.
Conflict of Interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Woodford, E.C., McLay, L., Blampied, N.M. et al. Less Restrictive Behavioral Interventions for Sleep Problems in Children with Neurodevelopmental Disorders: A Single Case Feasibility Study. J Dev Phys Disabil 35, 647–682 (2023). https://doi.org/10.1007/s10882-022-09872-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10882-022-09872-7