Abstract
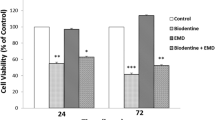
The primary objective of this study was to assess the biological effects of a new dentine substitute based on Ca3SiO5 (Biodentine™) for use in pulp-capping treatment, on pseudo-odontoblastic (MDPC-23) and pulp (Od-21) cells. The secondary objective was to evaluate the effects of Biodentine and mineral trioxide aggregate (MTA) on gene expression in cultured spheroids. We used the acid phosphatase assay to compare the biocompatibility of Biodentine and MTA. Cell differentiation was investigated by RT-qPCR. We investigated the expression of genes involved in odontogenic differentiation (Runx2), matrix secretion (Col1a1, Spp1) and mineralisation (Alp). ANOVA and PLSD tests were used for data analysis. MDPC-23 cells cultured in the presence of MTA had higher levels of viability than those cultured in the presence of Biodentine and control cells on day 7 (P = 0.0065 and P = 0.0126, respectively). For Od-21 cells, proliferation rates on day 7 were significantly lower in the presence of Biodentine or MTA than for control (P < 0.0001). Col1a1 expression levels were slightly lower in cells cultured in the presence of MTA than in those cultured in the presence of Biodentine and in control cells. Biodentine and MTA may modify the proliferation of pulp cell lines. Their effects may fluctuate over time, depending on the cell line considered. The observed similarity between Biodentine and MTA validates the indication for direct pulp-capping claimed by the manufacturers.



Similar content being viewed by others
References
Olsson H, Petersson K, Rohlin M. Formation of a hard tissue barrier after pulp cappings in humans. A systematic review. Int Endod J. 2006;39(6):429–42.
Al-Hiyasat AS, Barrieshi-Nusair KM, Al-Omari MA. The radiographic outcomes of direct pulp-capping procedures performed by dental students: a retrospective study. J Am Dent Assoc. 2006;137(12):1699–705.
Barthel CR, Rosenkranz B, Leuenberg A, Roulet JF. Pulp capping of carious exposures: treatment outcome after 5 and 10 years: a retrospective study. J Endod. 2000;26(9):525–8.
de Costa CAS, Duarte PT, de Souza PP, Giro EM, Hebling J. Cytotoxic effects and pulpal response caused by a mineral trioxide aggregate formulation and calcium hydroxide. Am J Dent. 2008;21(4):255–61.
Horsted-Bindslev P, Vilkinis V, Sidlauskas A. Direct capping of human pulps with a dentin bonding system or with calcium hydroxide cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96(5):591–600.
Nair PN, Duncan HF. Pitt Ford TR, Luder HU. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: a randomized controlled trial. Int Endod J. 2008;41(2):128–50.
Ford TRP, Torabinejad M, Abedi HR, Bakland LK, Kariyawasam SP. Using mineral trioxide aggregate as a pulp-capping material. J Am Dent Assoc. 1996;127(10):1491–4.
Bogen G, Kim JS, Bakland LK. Direct pulp capping with mineral trioxide aggregate: an observational study. J Am Dent Assoc. 2008;139(3):305–15.
Moghaddame-Jafari S, Mantellini M, Botero T, McDonald N, Nör J. Effect of ProRoot MTA on pulp cell apoptosis and proliferation in vitro. J Endod. 2005;31(5):387–91.
Paranjpe A, Zhang H, Johnson JD. Effects of mineral trioxide aggregate on human dental pulp cells after pulp-capping procedures. J Endod. 2010;36(6):1042–7.
Takita T, Hayashi M, Takeichi O, Ogiso B, Suzuki N, Otsuka K, et al. Effect of mineral trioxide aggregate on proliferation of cultured human dental pulp cells. Int Endod J. 2006;39(5):415–22.
Laurent P, Camps J, De Meo M, Dejou J, About I. Induction of specific cell responses to a Ca(3)SiO(5)-based posterior restorative material. Dent Mater. 2008;24(11):1486–94.
Laurent P, Camps J, About I. Biodentine (™) induces TGF-beta1 release from human pulp cells and early dental pulp mineralization. Int Endod J. 2012;45(5):439–48.
Koubi S, Elmerini H, Koubi G, Tassery H, Camps J. Quantitative evaluation by glucose diffusion of microleakage in aged calcium silicate-based open-sandwich restorations. Int J Dent. 2012;. doi:10.1155/2012/105863.
Koubi G, Colon P, Franquin JC, Hartmann A, Richard G, Faure MO, et al. Clinical evaluation of the performance and safety of a new dentine substitute, Biodentine, in the restoration of posterior teeth: a prospective study. Clin Oral Investig. 2013;17(1):243–9.
Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA. Multicellular tumor spheroids: an underestimated tool is catching up again. J Biotechnol. 2010;148(1):3–15.
Kunz-Schughart LA, Freyer JP, Hofstaedter F, Ebner R. The use of 3D cultures for high-throughput screening: the multicellular spheroid model. J Biomol Screen. 2004;9(4):273–85.
Shield K, Ackland ML, Ahmed N, Rice GE. Multicellular spheroids in ovarian cancer metastases: biology and pathology. Gynecol Oncol. 2009;113(1):143–8.
Kelm JM, Djonov V, Ittner LM, Fluri D, Born W, Hoerstrup SP, et al. Design of custom-shaped vascularized tissues using microtissue spheroids as minimal building units. Tissue Eng. 2006;12(8):2151–60.
Oudar O. Spheroids: relation between tumour and endothelial cells. Crit Rev Oncol Hematol. 2000;36(2):99–106.
Pampaloni F, Stelzer E. Three-dimensional cell cultures in toxicology. Biotechnol Genet Eng Rev. 2010;26:117–36.
Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ printing: tissue spheroids as building blocks. Biomaterials. 2009;30(12):2164–74.
Hanks CT, Sun ZL, Fang DN, Edwards CA, Wataha JC, Ritchie HH, et al. Cloned 3T6 cell line from CD-1 mouse fetal molar dental papillae. Connect Tissue Res. 1998;37(3–4):233–49.
Pang JL, Wu BL, He WX, Zhang YQ, Zhao HP, Xie ZH. Effect of antisense oligonucleotide against mouse dentine matrix protein 1 on mineralization ability and calcium ions metabolism in odontoblast-like cell line MDPC-23. Int Endod J. 2006;39(7):527–37.
Alno N, Jegoux F, Pellen-Mussi P, Tricot-Doleux S, Oudadesse H, Cathelineau G, et al. Development of a three-dimensional model for rapid evaluation of bone substitutes in vitro: effect of the 45S5 bioglass. J Biomed Mater Res A. 2010;95(1):137–45.
Friedrich J, Eder W, Castaneda J, Doss M, Huber E, Ebner R, et al. A reliable tool to determine cell viability in complex 3D culture: the acid phosphatase assay. J Biomol Screen. 2007;12(7):925–37.
Koulaouzidou EA, Economides N, Beltes P, Geromichalos G, Papazisis K. In vitro evaluation of the cytotoxicity of ProRoot MTA and MTA Angelus. J Oral Sci. 2008;50(4):397–402.
Zeferino EG, Bueno CE, Oyama LM, Ribeiro DA. Ex vivo assessment of genotoxicity and cytotoxicity in murine fibroblasts exposed to white MTA or white Portland cement with 15 % bismuth oxide. Int Endod J. 2010;43(10):843–8.
Rodriguez-Enriquez S, Gallardo-Perez JC, Aviles-Salas A, Marin-Hernandez A, Carreno-Fuentes L, Maldonado-Lagunas V, et al. Energy metabolism transition in multi-cellular human tumor spheroids. J Cell Physiol. 2008;216(1):189–97.
Lee SK, Lee SK, Lee SI, Park JH, Jang JH, Kim HW, et al. Effect of calcium phosphate cements on growth and odontoblastic differentiation in human dental pulp cells. J Endod. 2010;36(9):1537–42.
De Deus G, Ximenes R, Gurgel-Filho ED, Plotkowski MC, Coutinho-Filho T. Cytotoxicity of MTA and Portland cement on human ECV 304 endothelial cells. Int Endod J. 2005;38(9):604–9.
Howes AL, Chiang GG, Lang ES, Ho CB, Powis G, Vuori K, et al. The phosphatidylinositol 3-kinase inhibitor, PX-866, is a potent inhibitor of cancer cell motility and growth in three-dimensional cultures. Mol Cancer Ther. 2007;6(9):2505–14.
Paranjpe A, Smoot T, Zhang H, Johnson JD. Direct contact with mineral trioxide aggregate activates and differentiates human dental pulp cells. J Endod. 2011;37(12):1691–5.
Zhao X, He W, Song Z, Tong Z, Li S, Ni L. Mineral trioxide aggregate promotes odontoblastic differentiation via mitogen-activated protein kinase pathway in human dental pulp stem cells. Mol Biol Rep. 2012;39(1):215–20.
Zanini M, Sautier JM, Berdal A, Simon S. Biodentine induces immortalized murine pulp cell differentiation into odontoblast-like cells and stimulates biomineralization. J Endod. 2012;38(9):1220–6.
Peng W, Liu W, Zhai W, Jiang L, Li L, Chang J, et al. Effect of tricalcium silicate on the proliferation and odontogenic differentiation of human dental pulp cells. J Endod. 2011;37(9):1240–6.
Komori T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 2010;339(1):189–95.
Helder MN, Bronckers AL, Woltgens JH. Dissimilar expression patterns for the extracellular matrix proteins osteopontin (OPN) and collagen type I in dental tissues and alveolar bone of the neonatal rat. Matrix. 1993;13(5):415–25.
Linde A, Goldberg M. Dentinogenesis. Crit Rev Oral Biol Med. 1993;4(5):679–728.
Yokose S, Kadokura H, Tajima Y, Fujieda K, Katayama I, Matsuoka T, et al. Establishment and characterization of a culture system for enzymatically released rat dental pulp cells. Calcif Tissue Int. 2000;66(2):139–44.
Acknowledgments
The authors thank Dr. J.E. Nör and Dr. S. Simon for providing the cell lines used in this study. The authors wish to thank Pr. P. Colon and Pr. J-M. Vulcain for their assistance and wise advice. We thank Septodont (France) and Dentsply Maillefer (France) for providing the biomaterials.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pérard, M., Le Clerc, J., Meary, F. et al. Spheroid model study comparing the biocompatibility of Biodentine and MTA. J Mater Sci: Mater Med 24, 1527–1534 (2013). https://doi.org/10.1007/s10856-013-4908-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-013-4908-3