Abstract
Background
Clinical activity and quality of life (QOL) indices assess disease activity in Crohn’s disease (CD) and ulcerative colitis (UC). However, a paucity of data exists on the validity of these indices according to disease characteristics.
Aims
To examine the correlation between QOL and clinical activity indices and endoscopic disease activity according to disease characteristics.
Methods
We used a prospective registry to identify CD and UC patients ≥18 years old with available information on Short Inflammatory Bowel Disease Questionnaire scores (SIBDQ), Harvey–Bradshaw Index (HBI) and simple endoscopic scores for CD (SES-CD), and Simple Clinical Colitis Activity Index (SCCAI) and Mayo endoscopic score for UC. We used Spearman rank correlations to calculate correlations between indices and Fisher transformation to compare correlations across disease characteristics.
Results
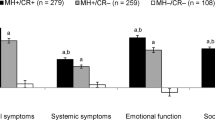
Among 282 CD patients, we observed poor correlation between clinical activity and QOL indices to SES-CD with no differences in correlation according to disease characteristics. Conversely, among 226 UC patients, clinical activity and QOL had good correlation to Mayo endoscopic score (r = 0.55 and −0.56, respectively) with better correlations observed with left-sided versus extensive colitis (r = 0.73 vs. 0.45, p = 0.005) and shorter duration of disease (r = 0.61 vs. 0.37, p = 0.04).
Conclusions
Our data suggest good correlation between SCCAI and endoscopic disease activity in UC, particularly in left-sided disease. Poor correlations between HBI or SIBDQ and SES-CD appear to be consistent across different disease phenotypes.




Similar content being viewed by others
References
Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124.
Alonso A, Domenech E, Julia A, et al. Identification of risk loci for Crohn’s disease phenotypes using a genome-wide association study. Gastroenterology. 2015;148:794–805.
Ho GT, Nimmo ER, Tenesa A, et al. Allelic variations of the multidrug resistance gene determine susceptibility and disease behavior in ulcerative colitis. Gastroenterology. 2005;128:288–296.
Veloso FT, Ferreira JT, Barros L, Almeida S. Clinical outcome of Crohn’s disease: analysis according to the Vienna classification and clinical activity. Inflamm Bowel Dis. 2001;7:306–313.
Schreiber S, Reinisch W, Colombel JF, et al. Subgroup analysis of the placebo-controlled CHARM trial: increased remission rates through 3 years for adalimumab-treated patients with early Crohn’s disease. J Crohn’s Colitis. 2013;7:213–221.
Hoie O, Wolters F, Riis L, et al. Ulcerative colitis: patient characteristics may predict 10-yr disease recurrence in a European-wide population-based cohort. Am J Gastroenterol. 2007;102:1692–1701.
Gerich ME, McGovern DP. Towards personalized care in IBD. Nat Rev Gastroenterol Hepatol. 2014;11:287–299.
Casellas F, Lopez-Vivancos J, Casado A, Malagelada JR. Factors affecting health related quality of life of patients with inflammatory bowel disease. Qual Life Res. 2002;11:775–781.
Pineton de Chambrun G, Peyrin-Biroulet L, Lemann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010;7:15–29.
Colombel JF, Rutgeerts PJ, Sandborn WJ, et al. Adalimumab induces deep remission in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:414–422.
Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512.
Voiosu T, Bengus A, Dinu R, et al. Rapid fecal calprotectin level assessment and the SIBDQ score can accurately detect active mucosal inflammation in IBD patients in clinical remission: a prospective study. J Gastrointest Liver Dis JGLD. 2014;23:273–278.
Dhanda AD, Creed TJ, Greenwood R, Sands BE, Probert CS. Can endoscopy be avoided in the assessment of ulcerative colitis in clinical trials? Inflamm Bowel Dis. 2012;18:2056–2062.
af Björkesten CG, Nieminen U, Turunen U, Arkkila P, Sipponen T, Färkkilä M. Surrogate markers and clinical indices, alone or combined, as indicators for endoscopic remission in anti-TNF-treated luminal Crohn’s disease. Scand J Gastroenterol. 2012;47:528–537.
Falvey JD, Hoskin T, Meijer B, et al. Disease activity assessment in IBD: clinical indices and biomarkers fail to predict endoscopic remission. Inflamm Bowel Dis. 2015;21:824–831.
Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Farkkila M. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008;14:40–46.
Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr. 1991;12:439–447.
Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007;133:423–432.
Hinkle DE, Wiersma W, Jurs SG. Applied statistics for the behavioral sciences. 5th ed. Boston, MA: Houghton Mifflin; 2003.
Fisher RA. Biometrika. Eugen Rev. 1916;8:62–64.
Fisher RA. Frequency distribution of the values of the correlation coefficient in samples from an indefinitely large population. Biometrika. 1925;10:507–521.
Peyrin-Biroulet L, Bressenot A, Kampman W. Histologic remission: the ultimate therapeutic goal in ulcerative colitis? Clin Gastroenterol Hepatol. 2014;12:929–934.
Irvine EJ, Feagan B, Rochon J, et al. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn’s Relapse Prevention Trial Study Group. Gastroenterology. 1994;106:287–296.
Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514.
Casellas F, Alcalá M-J, Prieto L, Miró J-RA, Malagelada J-R. Assessment of the influence of disease activity on the quality of life of patients with inflammatory bowel disease using a short questionnaire. Am J Gastroenterol. 2004;99:457–461.
Seo M, Okada M, Maeda K, Oh K. Correlation between endoscopic severity and the clinical activity index in ulcerative colitis. Am J Gastroenterol. 1998;93:2124–2129.
Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194–1201.
Khanna R, Bouguen G, Feagan BG, et al. A systematic review of measurement of endoscopic disease activity and mucosal healing in Crohn’s disease: recommendations for clinical trial design. Inflamm Bowel Dis. 2014;20:1850–1861.
Samaan MA, Mosli MH, Sandborn WJ, et al. A systematic review of the measurement of endoscopic healing in ulcerative colitis clinical trials: recommendations and implications for future research. Inflamm Bowel Dis. 2014;20:1465–1471.
Ricanek P, Brackmann S, Perminow G, et al. Evaluation of disease activity in IBD at the time of diagnosis by the use of clinical, biochemical, and fecal markers. Scand J Gastroenterol. 2011;46:1081–1091.
Casellas F, Vivancos JL, Sampedro M, Malagelada J-R. Relevance of the phenotypic characteristics of Crohn’s disease in patient perception of health-related quality of life. Am J Gastroenterol. 2005;100:2737–2742.
Shen B, Fazio VW, Remzi FH, et al. Clinical features and quality of life in patients with different phenotypes of Crohn’s disease of the ileal pouch. Dis Colon Rectum. 2007;50:1450–1459.
Kiss LS, Papp M, Lovasz BD, et al. High-sensitivity C-reactive protein for identification of disease phenotype, active disease, and clinical relapses in Crohn’s disease: a marker for patient classification? Inflamm Bowel Dis. 2012;18:1647–1654.
Henriksen M, Jahnsen J, Lygren I, et al. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut. 2008;57:1518–1523.
Charpentier C, Salleron J, Savoye G, et al. Natural history of elderly-onset inflammatory bowel disease: a population-based cohort study. Gut. 2014;63:423–432.
Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395.
Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65.
Williet N, Sandborn WJ, Peyrin-Biroulet L. Patient-reported outcomes as primary end points in clinical trials of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014;12:1246–1256.
Bodger K, Ormerod C, Shackcloth D, et al. Development and validation of a rapid, generic measure of disease control from the patient’s perspective: the IBD-Control questionnaire. Gut. 2014;63:1092–1102.
Kappelman MD, Long MD, Martin C, et al. Evaluation of the patient-reported outcomes measurement information system in a large cohort of patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2014;12:1315–1323.
Loftus EV Jr, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR. Crohn’s disease in Olmsted County, Minnesota, 1940–1993: incidence, prevalence, and survival. Gastroenterology. 1998;114:1161–1168.
Loftus EV Jr, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR. Ulcerative colitis in Olmsted County, Minnesota, 1940–1993: incidence, prevalence, and survival. Gut. 2000;46:336–343.
Yang D-H, Yang S-K, Park SH, et al. Usefulness of C-reactive protein as a disease activity marker in Crohn’s disease according to the location of disease. Gut Liver. 2015;9:80.
Goldman CD, Kodner IJ, Fry RD, MacDermott RP. Clinical and operative experience with non-Caucasian patients with Crohn’s disease. Dis Colon Rectum. 1986;29:317–321.
Ghazi LJ, Lydecker AD, Patil SA, Rustgi A, Cross RK, Flasar MH. Racial differences in disease activity and quality of life in patients with Crohn’s disease. Dig Dis Sci. 2014;59:2508–2513.
Yarur AJ, Abreu MT, Salem MS, Deshpande AR, Sussman DA. The impact of Hispanic ethnicity and race on post-surgical complications in patients with inflammatory bowel disease. Dig Dis Sci. 2014;59:126–134.
Acknowledgments
ST was involved in the study concept and design, analysis and interpretation of the data, drafting of the manuscript, and critical revision of the manuscript. KOS was involved in the statistical analysis and drafting of the manuscript. DKL and PS were involved in acquisition of data. DSP was involved in the study concept and design and critical revision of the manuscript for important intellectual content. HCS, DN, VY, ANN were involved acquisition of data and critical revision of the manuscript for important intellectual content. HK was involved in the study concept and design, analysis and interpretation of the data, and critical revision of the manuscript.
Funding
This work is supported by a career development award from the American Gastroenterological Association (AGA) and the National Institute of Diabetes and Digestive and Kidney Diseases (K23 DK099681 to HK) along with a grant from the National Institutes of Health (K23 DK097142 to ANN).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Dr. Ananthakrishnan is a member of the scientific advisory board for Exact Sciences, AbbVie, and Cubist pharmaceuticals. Dr. Khalili has received consultant fee from AbbVie. Dr. Yajnik has received consulting fees from NPS, Janssen Pharmaceuticals, and UCB. None of the authors had any personal disclosures.
Additional information
Sasha Taleban and Kathleen O. Stewart have contributed equally to the manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Taleban, S., Stewart, K.O., Li, D.K. et al. Clinical Activity and Quality of Life Indices Are Valid Across Ulcerative Colitis But Not Crohn’s Disease Phenotypes. Dig Dis Sci 61, 2627–2635 (2016). https://doi.org/10.1007/s10620-016-4180-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-016-4180-8