Abstract
Valve size selection for transcatheter aortic valve replacement (TAVR) is currently based on cardiac CT-scan. At variance with patient-specific computer simulation, this does not allow the assessment of the valve-host interaction. We aimed to compare clinical valve size selection and valve size selection by an independent expert for computer simulation. A multicenter retrospective analysis of valve size selection by the physician and the independent expert in 141 patients who underwent TAVR with the self-expanding CoreValve or Evolut R. Baseline CT-scan was used for clinical valve size selection and for patient-specific computer simulation. Simulation results were not available for clinical use. Overall true concordance between clinical and simulated valve size selection was observed in 47 patients (33%), true discordance in 15 (11%) and ambiguity in 79 (56%). In 62 (44%, cohort A) one valve size was simulated whereas two valve sizes were simulated in 79 (56%, cohort B). In cohort A, concordance was 76% and discordance was 24%; a smaller valve size was selected for simulation in 10 patients and a larger in 5. In cohort B, a different valve size was selected for simulation in all patients in addition to the valve size that was used for TAVR. The different valve size concerned a smaller valve in 45 patients (57%) and a larger in 34 (43%). Selection of the valve size differs between the physician and the independent computer simulation expert who used the same source of information. These findings indicate that valve sizing in TAVR is still more intricate than generally assumed.
Similar content being viewed by others
Introduction
Transcatheter aortic valve replacement (TAVR) is an accepted treatment in patients with severe aortic stenosis at a high or intermediate operative risk [1,2,3,4,5]. At present, different valve types and sizes are available allowing optimal transcatheter valve performance and clinical outcome in a wide range of patients [5,6,7,8]. Availability of different valves and sizes implies that choosing the valve that best fits the individual patient has become more challenging in particular as outcome of TAVR depends among others on device-host interaction. Currently, computed tomography (CT) scan of the heart is the standard method and recommended for selection of the valve size [9]. Yet, this does not allow the prediction of the mechanical interaction and precise outcome between the device and host. For that purpose, a dedicated computer simulation model has been developed and validated to predict case-by-case calcium displacement, presence and severity of aortic regurgitation (AR) and conduction disturbances post TAVR [10,11,12,13]. The purpose of this study was to compare transcatheter valve size selection between the physician for TAVR and the independent expert for the purpose of patient-specific computer simulation.
Patients and methods
Study population
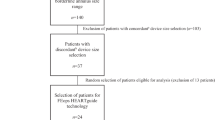
The study population consisted of 141 patients who had undergone TAVR with the self-expanding Medtronic valve (CoreValve [MCS] or Evolut R) because of native tricuspid aortic stenosis and in whom patient-specific computer simulation was performed for the assessment and prediction of valve performance. The information of the computer simulation was not available for clinical application. The valve size selected for the clinical implantation (TAVR) was decided by the physician based upon pre-procedural multi-slice computed tomography (MSCT) as previously described [14]. In all patients and participating centers dedicated software (i.e., 3 Mensio, Pie Medical Imaging BV, Maastricht, the Netherlands) was used for quantitative analysis of the aortic root [15].
All patients provided written informed consent for TAVR and data collection. Clinical data was extracted from local databases. The study was conducted in accordance with the principles of the Declaration of Helsinki and did not fall under the scope of the Medical Research Involving Human Subjects Act per EMC Institutional Review Board (MEC nr. 2019-0442).
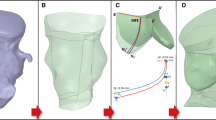
Patient-specific computer simulation was performed by an independent institution (FEops, Ghent, Belgium) by first creating a virtual model of each patient’s anatomy by segmentation and 3D reconstruction of the aortic root (including the calcified native leaflets and outflow tract [LVOT]) based on pre-procedural anonymized MSCT (Mimics Software, Materialise, Leuven, Belgium) [10,11,12,13]. All steps performed during TAVR were respected during simulation such as eventual pre- or postdilatation and valve type (i.e., MCS, Evolut R) but not valve size (Abaqus/Explicit finite element solver, Dassault Systèmes, Paris, France). The valve size used during simulation was decided by the independent simulation expert using the aortic annulus dimensions and the manufacturer’s matrix of sizing. As the latter contains precise cut-off values for each MCS and Evolut R valve size, a margin of ± 2% was used for each cut off value (i.e., grey zone of valve size selection). In case a patient had an aortic annulus dimension that falls within the grey zone, 2 sizes were simulated. During simulation, 3 levels of depth of implantation were executed; 2 mm below the annulus (high implant), 5 mm below the annulus (medium depth of implantation) and 8 mm below the annulus (low implant).
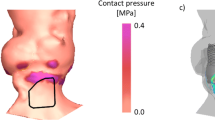
Outcome measures of the simulation were: (1) coronary obstruction, (2) aortic regurgitation (AR) and (3) contact pressure of the frame within a predefined area below the annulus [10,11,12,13]. Coronary obstruction was defined by measuring the distance between the closest native aortic leaflet calcium nodule and the center of the left and right coronary ostium post TAVR (i.e., coronary obstruction present if distance: 0 mm) [10]. Severity of AR post TAVR was measured using computational fluid dynamics and was expressed in ml/s. A cut-off value of 16.0 ml/s has been reported to correspond to ≥ moderate AR [11]. Contact pressure exerted by the frame in the region of the LVOT hosting the conduction tissue was expressed as maximum contact pressure (MPa) and area (i.e., contact pressure index, %). Cut-off values of respectively 0.39 MPa and 14% were earlier found to correlate with the occurrence of new left bundle branch block (LBBB) or high degree atrio-ventricular block (high degree AVB) [13].
Clinical outcome measures (after TAVR) were: (1) coronary obstruction defined by TIMI 0 flow during contrast angiography immediately post TAVR, (2) AR assessed by transthoracic echocardiography before discharge [VARC-2 criteria, 16] and (3) LBBB or high degree AVB (i.e., Mobitz II or 3rd degree AV block) defined by 12-lead ECG before discharge [17].
Statistical analysis
The main analysis consisted of the assessment of the agreement between the valve size selected for simulation and TAVR and, the comparison between the predicted (simulation) and clinical (TAVR) outcomes, namely (1) coronary obstruction, (2) AR and (3) new LBBB or high degree AVB in relation to maximum contact pressure and area after TAVR. For the purpose of this study, the simulation-derived outcome measures at a medium depth of implantation were used.
Normality of distributions was tested by the Kolmogorov–Smirnov test. Continuous variables are shown as mean ± standard deviation (SD) or medians [interquartile ranges (IQR) 25–75%] as appropriate. Categorical variables are expressed as frequencies and percentages. Comparison between predicted and observed coronary obstruction and AR post TAVI (≥ grade 2) was made by means of the McNemar’s test.
Results
Study population
The baseline demographic and procedural characteristics of the 141 patients are summarized in Table 1. The median age was 82 (78–85) years and 50% were male. In 115 patients (82%) a MCS valve was implanted while 26 (18%) received an Evolut R valve. With respect to valve size, a 29 mm valve was implanted in 92 patients (62%), a 26 mm in 40 (28%) and a 31 mm in 9 (6%). In all patients some degree of oversizing was observed, 18 ± 7% when using the perimeter and 20 ± 7% when using the mean diameter.
Findings
In 62 out of the 141 patients (44%) one valve size was simulated (cohort A), 2 valve sizes were simulated in 79 patients (56%, cohort B). In cohort A (n = 62), concordance (i.e., same valve size selected for simulation and TAVR) was 76% (47 patients). Discordance was observed in 15 patients (24%, Table 2); a smaller valve was selected for simulation in 10 patients and a larger in 5. In cohort B (n = 79, Table 3), a different valve size was selected for simulation in all patients in addition to the valve size that was used for TAVR; a smaller valve was selected for simulation in 45 patients (57%) and a larger in 34 (43%). This means that overall true concordance (same valve size selection for simulation and TAVR by independent expert or physician) was observed in 33% of patients, true discordance in 11% and ambiguity (i.e., two valve sizes selected for simulation and thus a priori considered eligible for TAVR) in 56% of patients. In case of discordance and ambiguity, a smaller valve was used for simulation in 39% of patients and a larger in 28%. In case of discordant valve size selection in cohort A (n = 15), the degree of oversizing was 18 ± 7% when using the perimeter and 20 ± 7% when using the mean diameter. It was 17 ± 8 and 15 ± 7%, respectively in cohort B (different valve size used for simulation).
Outcome cohort A
In correspondence with the clinical observation, simulation did not predict any case of coronary obstruction (Table 2). In case of discordant valve size selection, the simulation predicted a higher degree of AR post TAVR [16.6 (11.3–23.7) ml/s] and (based upon the recently validated cutoff-value of AR grade ≥ 2) a higher prevalence of AR ≥ grade 2 (60%) in comparison with concordant valve size selection [14.0 (6.6–24.4) ml/s and 36%), albeit that in both situations the predicted AR exceeded the observed AR. Predicted AR ≥ grade 2 was significantly higher compared to observed in case of concordant valve size selection (p = 0.002, Table 2). The predicted maximum contact pressure and contact pressure index were lower in case of discordant valve size selection (Table 2). The prevalence of clinical (i.e., observed) new LBBB and high degree AVB after TAVR were similar between con- and discordant valve size selection.
Outcome cohort B
Similar to cohort A, coronary obstruction was neither predicted nor observed (Table 3). In case of simulation of a valve size different from TAVR, the simulation predicted a higher degree of AR post TAVR [15.4 [7.5–27.5]) ml/s] and a higher prevalence of AR ≥ grade 2 (48%) in comparison with concordant valve size selection [12.2 (5.1–22.1) ml/s and 42%]. Predicted AR ≥ grade 2 was higher than observed in both concordant and discordant valve size selection (respectively p = 0.007 and p = 0.001). No difference in predicted maximum contact pressure and contact pressure index were seen when using the same or different valve size for simulation (Table 3).
Discussion
When comparing valve size selection by the physician (using MSCT and dedicated software for quantitative analysis of the aortic root) with valve size selection for patient-specific computer simulation, we found true concordance in 33% of the patients, true discordance in 11% and ambiguity in 56%. Moreover, a smaller valve was selected for simulation in 39% of the patients and a larger in 28%. These findings indicate that valve size selection in TAVR still is more intricate than generally assumed even when using MSCT that is the standard imaging modality for TAVR planning including valve size selection [9].
These findings need to be interpreted against the following; firstly, the physician generally adheres to the cut-off values for sizing proposed by the manufacturer’s matrix of sizing albeit with some degree of oversizing while a margin of 2% at each cut-off value in two directions was used for the selection of valve size(s) for computer simulation. This margin of which its value (i.e., 2%) was arbitrarily chosen was introduced as the application of strict cut-off criteria in clinical practice is often not realistic or preferable and lacks a sound pathophysiologic basis. The use of such a margin or grey zone may explain the observation that a smaller valve size was used for simulation in 39% of the patients and a larger in 28% in the present analysis.
There are distinct differences between clinical valve size selection (physician) and valve size selection based upon computer simulation. In the former, the physician selects the valve size upon the quantitative assessment of the dimensions of the aortic root while in the latter–that starts with MSCT analysis–the effects of the device-host interaction (e.g., coronary obstruction, AR post TAVR and conduction abnormalities) are taken into account. The device-host interaction is based upon the integration of the dimensions of the device and host and, their biomechanical properties. Tissue biomechanical properties are derived from experimental data subsequently refined during so-called training of the computer model during validation studies [10,11,12,13]. Biomechanical properties of the frame are derived from standard in vitro testing of the stress–strain relationship [18]. Of note, the biomechanical properties of the nitinol frame of the herein used valves are such that its hysteresis loop has a plateau (i.e., no change in radial force during a certain phase of frame compression or expansion) with some degree of overlap between two adjacent valve sizes. This implies that–when a physician decides to oversize to ensure proper anchoring and to avoid PVL–such a strategy does not necessarily translates into a higher contact pressure exerted by the frame on the LVOT and consequently new conduction abnormalities. This is supported by the observation that the predicted maximum contact pressure and contact pressure index were similar when using the same or different valve size for simulation in cohort B. However, a higher predicted maximum contact pressure and contact pressure index is seen in concordant cohort A versus discordant cohort A which we believe is because majority (10/15) of simulations in discordant valve size selection was performed with a smaller valve compared to clinical practice. A smaller implanted valve is expected to result in less contact pressure. With regard to AR ≥ grade 2, a higher frequency was predicted than observed in cohort A (concordant) and cohort B (concordant and discordant) which may be the result of AR post TAVR quantification by means of echocardiography based on the VARC 2 method since it is known that echocardiography is inferior to magnetic resonance imaging (MRI) for the assessment of AR and underestimates the actual degree of AR [19].
The selection of the valve size (and type) that best fits the individual patient is mandatory as a patient-specific or -tailored approach ensures maximum safety and efficacy. This is in particular important with the increasing number of sizes and types of valves that are and will be available in clinical practice in combination with the fact that the aortic root differs from patient to patient. We acknowledge that in this analysis, we concentrated on one single valve type (i.e., the self-expanding MCS and Evolut R) and did not include the balloon-expandable and mechanically expanding valves. Yet, conceptually the role of patient-specific computer simulation in clinical practice is self-explanatory. This, however, needs to be proven by appropriately designed studies such as RCT (physician vs. simulation driven valve size and type selection) and implies that the software keeps track with the rapid development of novel valve technologies and sizes. Given the rapid development of valve technologies that effectively address the vexing issues of TAVR (AR in particular), patient-specific computer simulation may have in particular a clinical value in cases of uncertainty in valve size selection for instance due to the strict sizing criteria proposed by the manufacturer in combination with observer variability in MSCT analysis. It may especially have a clinical added value in the selection of the valve type that best fits the individual patient.
Limitations
This study concerns an exploratory analysis of the comparison between valve size selection between the physician for TAVR and the independent expert for the purpose of patient-specific computer simulation. The study design consisted of a retrospective analysis which may introduce bias. Predicted outcomes represent the situation directly post TAVR and no conclusions on long term can be drawn based on this study. In addition to the limitations discussed above, the sample and the fact that only three centers participated need to be considered and affect generalizability.
Conclusion
Selection of the size of the self-expanding valve by the physician for TAVR and the independent expert for patient-specific computer simulation differs substantially. Concordance was found in only 33% of the patients, true discordance in 11% and ambiguity in 56%. A smaller valve was selected for simulation in 39% of the patients and a larger in 28%. These findings indicate that valve sizing in TAVR is still more intricate than generally assumed.
References
Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ, PARTNER Trial Investigators (2011) Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 364(23):2187–2198
Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, Fontana GP, Dewey TM, Thourani VH, Pichard AD, Fischbein M, Szeto WY, Lim S, Greason KL, Teirstein PS, Malaisrie SC, Douglas PS, Hahn RT, Whisenant B, Zajarias A, Wang D, Akin JJ, Anderson WN, Leon MB, PARTNER Trial Investigators (2012) Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 366(18):1686–1695
Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S, PARTNER Trial Investigators (2010) Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 363(17):1597–1607
Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, Thourani VH, Babaliaros VC, Webb JG, Herrmann HC, Bavaria JE, Kodali S, Brown DL, Bowers B, Dewey TM, Svensson LG, Tuzcu M, Moses JW, Williams MR, Siegel RJ, Akin JJ, Anderson WN, Pocock S, Smith CR, Leon MB, PARTNER Trial Investigators (2012) Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 366(18):1696–1704
Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG, PARTNER 2 Investigators (2016) Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 374(17):1609–1620
Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, Chetcuti S, Gleason T, Heiser J, Lange R, Merhi W, Oh JK, Olsen PS, Piazza N, Williams M, Windecker S, Yakubov SJ, Grube E, Makkar R, Lee JS, Conte J, Vang E, Nguyen H, Chang Y, Mugglin AS, Serruys PW, Kappetein AP, SURTAVI Investigators (2017) Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 376(14):1321–1331
Barbanti M, Buccheri S, Rodés-Cabau J, Gulino S, Généreux P, Pilato G, Dvir D, Picci A, Costa G, Tamburino C, Leon MB, Webb JG (2017) Transcatheter aortic valve replacement with new-generation devices: a systematic review and meta-analysis. Int J Cardiol 15(245):83–89
Abdel-Wahab M, Richardt G (2014) Selection of TAVI prostheses: do we really have the CHOICE? EuroIntervention 10(Suppl U):U28–U34
Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL, ESC Scientific Document Group (2017) 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 38(36):2739–2791
Schultz C, Rodriguez-Olivares R, Bosmans J, Lefèvre T, De Santis G, Bruining N, Collas V, Dezutter T, Bosmans B, Rahhab Z, El Faquir N, Watanabe Y, Segers P, Verhegghe B, Chevalier B, van Mieghem N, De Beule M, Mortier P, de Jaegere P (2016) Patient-specific image-based computer simulation for the prediction of valve morphology and calcium displacement after TAVI with the medtronic CoreValve and the Edwards SAPIEN valve. EuroIntervention 11:1044–1052
de Jaegere P, De Santis G, Rodriguez-Olivares R, Bosmans J, Bruining N, Dezutter T, Rahhab Z, El Faquir N, Collas V, Bosmans B, Verhegghe B, Ren C, Geleinse M, Schultz C, van Mieghem N, De Beule M, Mortier P (2016) Patient-specific computer modeling to predict aortic regurgitation after transcatheter aortic valve replacement. JACC Cardiovasc Interv 9:508–512
El Faquir N, Ren B, Van Mieghem NM, Bosmans J, de Jaegere PP (2017) Patient-specific computer modelling – its role in the planning of transcatheter aortic valve implantation. Neth Heart J 25(2):100–105
Rocatello G, El Faquir N, De Santis G, Iannaccone F, Bosmans J, De Backer O, Sondergaard L, Segers P, De Beule M, de Jaegere P, Mortier P (2018) Patient-specific computer simulation to elucidate the role of contact pressure in the development of new conduction abnormalities after catheter-based implantation of a self-expanding aortic valve. Circ Cardiovasc Interv 11(2):e005344
Schultz C, Moelker A, Tzikas A, Piazza N, de Feyter P, van Geuns RJ, Serruys PW, Krestin GP, de Jaegere P (2010) The use of MSCT for the evaluation of the aortic root before transcutaneous aortic valve implantation: the Rotterdam approach. EuroIntervention 6(4):505–511
de Vaan J, Verstraeten L, de Jaegere P, Schultz C (2012) The 3mensio Valves™ multimodality workstation. EuroIntervention 7(12):1464–1469
Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodés-Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB (2012) Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol 60(15):1438–1454
Surawicz B, Childers R, Deal BJ, Gettes LS, Bailey JJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, Rautaharju PM, van Herpen G, Wagner GS, Wellens H, American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology, American College of Cardiology Foundation; Heart Rhythm Society (2009) AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 53(11):976–981
Tzamtzis S, Viquerat J, Yap J, Mullen MJ, Burriesci G (2013) Numerical analysis of the radial force produced bythe Medtronic-CoreValve and Edwards-SAPIEN after transcatheter aortic valve implantation (TAVI). Med Eng Phys 35(1):125–130
Ribeiro HB, Le Ven F, Larose E, Dahou A, Nombela-Franco L, Urena M, Allende R, Amat-Santos I, Ricapito Mde L, Thébault C, Clavel MA, Delarochelliére R, Doyle D, Dumont E, Dumesnil JG, Pibarot P, Rodés-Cabau J (2014) Cardiac magnetic resonance versus transthoracic echocardiography for the assessment and quantification of aortic regurgitation in patients undergoing transcatheter aortic valve implantation. Heart 100(24):1924–1932
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Peter Mortier is shareholder of FEops NV.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
El Faquir, N., Rocatello, G., Rahhab, Z. et al. Differences in clinical valve size selection and valve size selection for patient-specific computer simulation in transcatheter aortic valve replacement (TAVR): a retrospective multicenter analysis. Int J Cardiovasc Imaging 36, 123–129 (2020). https://doi.org/10.1007/s10554-019-01688-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-019-01688-5