Abstract
Prior myocardial infarction (MI) is associated with increased mortality and is prevalent in certain high risk patient groups. Electrocardiogram may be used in diagnosis, however, sensitivity is limited, thus non-invasive imaging techniques may improve diagnosis. We investigated whether global longitudinal strain (GLS) and longitudinal strain parameters are reduced in patients with prior MI but preserved left ventricular ejection fraction (LVEF). The study included 40 clinical patients with prior MI occurring >3 months previously (defined as subendocardial hyperenhancement on late Gadolinium enhancement imaging) with LVEF ≥ 55% and 40 controls matched for age and LVEF. GLS, global longitudinal strain rate (GLSR) and early diastolic longitudinal strain rate (GLSRe) were measured from cine imaging feature tracking analysis. Presence of wall motion abnormality (WMA) and minimum systolic wall thickening (SWT) were calculated from cine imaging. GLS was −17.3 ± 3.7% in prior MI versus −19.3 ± 1.9% in controls (p = 0.012). GLSR was −88.0 ± 33.7%/s in prior MI versus −103.3 ± 26.5%/s in controls (p = 0.005). GLSRe was 76.4 ± 28.4%/s in prior MI versus 95.5 ± 26.0%/s in controls (p = 0.001). GLS accurately identified prior MI [AUC 0.662 (95% CI 0.54–0.785) p = 0.012] whereas WMA [AUC 0.500 (95% CI 0.386–0.614) p = 1.0] and minimum SWT [AUC 0.609 (95% CI 0.483–0.735) p = 0.093] did not. GLS, GLSR and GLSRe are reduced in prior MI with preserved LVEF. Normal LVEF and lack of WMA cannot exclude prior MI. Prior MI should be considered when reduced GLS, GLSR or GLSRe are detected by non-invasive imaging.
Similar content being viewed by others
Introduction
Prior myocardial infarction (MI) is defined as either the presence of pathological Q waves on an electrocardiogram (ECG), regional loss of myocardium on cardiovascular (CV) imaging in the absence of a non-ischemic cause or pathological findings supportive of prior MI [1]. Myocardial infarction is frequently unrecognized at the time of its occurrence, accounting for 20–40% of all prior MI in high risk populations diagnosed on ECG criteria [2, 3]. Cardiovascular magnetic resonance (CMR) late gadolinium enhancement (LGE) imaging offers a more sensitive means of diagnosis than ECG and is considered the reference standard non-invasive imaging technique for the detection of prior MI [4, 5]. Studies using this approach have suggested that unrecognized MI is more common than recognized prior MI in certain populations, with a prevalence of 18% in an elderly, community-based cohort [6].
The presence of unrecognized MI detected by LGE is associated with a tenfold increase in risk of CV mortality, which appears to be incremental to conventional clinical and imaging risk factors [7]. Recognition of the condition is therefore important and secondary prevention therapy aimed at reducing long-term CV risk is recommended when prior MI is diagnosed [8].
Longitudinal strain parameters theoretically have the potential to detect prior MI. Myocardial fibers situated in the left ventricular (LV) subendocardium contribute significantly to longitudinal LV contraction in systole [9]. These fibers are susceptible to ischemia and increased wall stress [10] and hence, prior MI affecting these fibers may lead to reductions in longitudinal strain values [11]. Global longitudinal strain (GLS) is a summation of myocardial deformation in the longitudinal plane during systole [12]. It is proposed to detect subclinical LV systolic function in a number of cardiomyopathies where LV ejection fraction (LVEF) is preserved [13]. Global longitudinal strain rate (GLSR) and early diastolic longitudinal strain rate (GLSRe) represent the rate of longitudinal systolic and early diastolic deformation [12] and are comparable to tissue Doppler derived S prime and E prime measurements [14]. CMR feature tracking is an alternative method of measuring these and other myocardial strain indices and uses post-processing software to rapidly obtain measurements from steady state free precession (SSFP) cine imaging.
GLS has been demonstrated to be reduced in patients with heart failure with reduced ejection fraction (HF-REF) due to chronic ischemic heart disease [15] and in the setting of acute MI [16]. However, GLS in patients with MI but preserved LVEF has not yet been investigated.
We hypothesized that patients with prior MI detected by LGE but preserved LVEF would have impaired GLS and longitudinal strain rates compared to matched normal controls.
Materials and methods
Study population
This was a single centre case control study involving 40 clinical patients with prior MI occurring >3 months previously and preserved LVEF (≥55%) and 40 controls matched for age and sex with LVEF (≥55%). Prior MI patients were retrospectively recruited from consecutive patients undergoing CMR for clinical reasons and selecting those with MI and preserved LVEF. Healthy controls were prospectively recruited volunteers with no history of cardiac disease. All participants were screened for CV risk factors by completion of a health questionnaire and from medical records.
Inclusion and exclusion criteria
Patients with significant arrhythmia (defined as uncontrolled tachycardia >100 bpm at the time of CMR study) were excluded. Prior MI patients with a history of acute MI or chest pain within the preceding 3 months were excluded on the basis that significant alterations to both myocardial scar burden and LV systolic function could have occurred in this time frame. Controls had no known history of CV disease.
CMR acquisition
All patients underwent CMR at either 1.5 T (Philips Ingenia) or 3.0 T (Philips Achieva) and controls at 3.0 T (Philips Achieva). Images were acquired with breath holding on end-expiration prior to contrast administration and prospectively gated using a 3-lead vector ECG.
Cine images were planned from the scout images and for every patient a 2 chamber (2Ch), 4 chamber (4Ch) and an LV short axis cine stack were acquired to ensure full coverage of the left ventricle. Typical image acquisition parameters for SSFP cine acquisitions were as follows: TR 2.6 ms, TE 1.3 ms, flip angle 40°, field of view 320 × 340 mm × 100 mm, voxel size 2 × 1.62 × 10 mm, 30 cardiac phases.
LGE imaging was performed in all patients with acquisitions of a short axis LV stack, 2Ch and 4Ch obtained 10 min after administration of 0.2 mmol/kg Gadolinium DTPA contrast (Gadovist, Bayer Schering) using inversion recovery-prepared T1 weighted echo. The optimal inversion time (TI) to null normal myocardial signal ascertained by the Look Locker approach. Between 10 and 12 short axis LV, 2Ch and 4Ch images were acquired for every patient. Further imaging with altered phase-encoding direction or systolic imaging were acquired when prior MI was suspected after initial imaging.
CMR analysis
All post processing analysis of CMR scans was performed using the same software (CVI 42, Circle Cardiovascular Imaging Calgary, Canada). LV contours were drawn manually at both end diastole and end systole on the LV short axis SSFP cine stack. LV papillary muscles were considered part of the LV cavity.
Percentage myocardial systolic wall thickening (SWT) was calculated from the end diastolic and end systolic contours as previously reported [17]. SWT was calculated for each LV segment based on the 16 segment AHA model. A cut-off value of 30% was used to define the presence of wall motion abnormality (WMA) in an individual LV segment [18]. The minimum value for each of the 16 LV segments in each patient was taken and used for comparison between prior MI patients and controls.
The presence of LGE in a subendocardial pattern suggestive of prior MI was determined independently by two physicians with over 4 years’ experience in CMR. Quantitative assessment of myocardial scar burden was performed using a threshold of 50% of the maximum intensity within the scar (full width half max method) which has been proposed as the most reproducible method for this purpose [19]. After optimization of brightness and contrast settings, manual delineation of two separate user-defined regions of interest (ROIs) were made on an LGE short axis slice where infarcted myocardium was present. One ROI was an area of hyperintense infarcted myocardium and a second ROI was drawn in remote myocardium containing no infarcted myocardium. Automated calculations for the remaining LV short axis LGE stack based on these two ROIs were then performed.
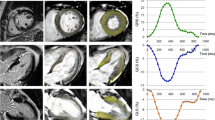
The strain parameters GLS, GLSR and GLSRe were calculated using feature tracking software from 4Ch and 2Ch SSFP cine acquisitions (Fig. 1). Prior to analysis, brightness and contrast settings were adjusted to allow optimization of endocardial and blood pool differentiation. The epicardial and endocardial borders were traced manually. The software then tracked the voxel features of the myocardium to quantify the motion of myocardium and compute strain values [20]. This process results in generation of values of percentage deformation in the longitudinal plane throughout the cardiac cycle (providing a value for GLS) as well as deformation rate throughout the cardiac cycle (providing values for GLSR and GLSRe).
Calculation of GLS using CMR feature tracking in a healthy control. Panel A 4Ch cine acquisition with manually contoured epicardial and endocardial borders. Panel B 2Ch cine acquisition with manually contoured epicardial and endocardial border. Panel C feature tracking of 4Ch cine acquisition. Panel D feature tracking of the 2Ch cine acquisition. Panel E graph showing GLS [x axis shows time (ms) and y axis shows deformation (%) in longitudinal plane]. Panel F graph showing longitudinal strain rate [x axis shows time (m/s) and y axis shows deformation rate (%/s)]
Statistical analysis and power calculation
Normality of data was tested using a Shapiro–Wilk test. Mean values ± SD are reported. Unpaired Student t test and Mann–Whitney U test were used as appropriate to compare continuous variables. Cut-off values to identify prior MI were derived from receiver-operating characteristic (ROC) curve analysis using Youden index giving maximum sensitivity and specificity. AUCs were compared by using validated methods described by DeLong et al. [21]. Multivariable linear regression was used for variables with a statistical significance of <0.1 on univariable linear regression. Intra and interobserver variability for GLS were tested on ten randomly selected healthy controls using coefficient of variation (CoV). All tests were two-sided and p < 0.05 was considered statistically significant.
Based on the pooled standard deviation of 2.8% 31 subjects are needed in each group to detect an absolute reduction of GLS by 2% in those with chronic MI (α = 0.8, significance = 0.05).
Results
A total of 40 prior MI and 40 healthy controls were recruited. Analysis was completed in all 40 patients in both groups, thus all were included in the final study sample. The prior MI patients were well-matched with controls for age and sex. Other variables including blood pressure, body mass index, LV mass, LV end diastolic volume (LVEDV) and LV systolic function were comparable across both groups. There were no statistically significant differences between any of these variables (Table 1).
Feature tracking parameters of myocardial strain
Feature tracking analysis was successfully performed in all prior MI patients and controls. Global longitudinal strain (Fig. 2) was significantly lower in prior MI patients than controls (−17.3 ± 3.7% versus −19.3 ± 1.9%, p = 0.012). Global longitudinal strain rate was significantly lower in prior MI patients versus controls (−88.0 ± 33.7% s−1 versus −103.3 ± 26.5% s−1 p = 0.005). Early diastolic longitudinal strain rate was also significantly lower in prior MI patients versus controls (76.4 ± 28.4% s−1 versus 95.5 ± 26.0% s−1, p = 0.001). GLS was not significantly different in prior MI patients scanned at 1.5 and 3.0 T (mean GLS at 1.5 T −18.0 ± 1.8 versus −17.2 ± 4.0 at 3.0 T, p = 0.61).
Box and whisker plot for GLS in prior MI and healthy controls. GLS values for all prior MI patients and healthy controls are shown as individual data points. For both groups, the horizontal line in the middle of the box demonstrates median values, the bottom of the box represents the 25th percentile and the top of the box represents the 75th percentile. The T-bar ‘whiskers’ represent the 95% confidence intervals
Quantitative systolic wall thickening
Quantitative systolic wall thickening analysis was possible in all prior MI patients and controls. There was no significant difference in minimum SWT in those with prior MI compared to controls (46.0 ± 18.3% versus 42.0 ± 14.1%, p = 0.093). There was no significant difference in the proportion of subjects with WMA defined as a segment with SWT < 30% [6/40 (15%) prior MI patients and 6/40 (15%) controls].
Receiver operator characteristic analysis
AUC for the ability of each longitudinal strain parameter to correctly identify prior MI were as follows (Fig. 3): GLS 0.662 (95% CI 0.540–0.785), p = 0.01, GLSR 0.684 (95% CI 0.566–0.802), p = 0.005 and for GLSRe 0.707 (95% CI 0.592–0.821), p = 0.001. By comparison, AUC for the ability of both presence of WMA and minimum SWT to correctly identify prior MI were lower at 0.500 (95% CI 0.386–0.614), p = 1.0 and 0.609 (95% CI 0.483–0.735), p = 0.09 respectively. Comparison of AUC values for all longitudinal strain parameters assessed showed significantly higher diagnostic accuracy for the detection of prior MI than WMA (GLS p = 0.02, GLSR p = 0.01 and GLSRe p = 0.001). Comparison of AUC values between longitudinal strain parameters and minimum SWT showed no statistical significance (GLS p = 0.59, GLSR p = 0.39 and GLSRe p = 0.29).
Scar quantitation
Scar quantitation was successfully performed in all prior MI patients. The range of absolute scar mass was wide at between 0.7 and 21.4 g. The overall mean absolute scar mass was 5.5 ± 4.3 g, equating to a relative percentage of LV mass of 4.9 ± 3.3%.
Sensitivity and specificity of feature tracking derived strain values
Two approaches were used in determining sensitivity and specificity for the correct identification of prior MI by the different longitudinal strain values assessed. Firstly, the cut-off value giving maximum area under the curve was determined for each variable. Secondly, the value giving maximum specificity was determined. Results are summarized in Table 2.
Univariable and multivariable regression analysis for GLS
Variables including patient demographics, risk factors for CVD and presence of prior MI were analyzed to determine univariable predictors of GLS (Table 3). Multivariable regression analysis revealed only prior MI to be an independent predictor of change in GLS.
Observer variability
On intraobserver analysis, mean GLS values by CMR feature tracking were similar at −20.3 and −19.6% (p = 0.60) and CoV 3.9%. On interobserver analysis, mean GLS values CMR feature tracking were again similar at −20.3% and −19.6 (p = 0.62) and CoV% 4.0%.
Discussion
We have found that in patients with preserved LVEF and prior MI there is impairment of GLS, GLSR and GLSRe. Furthermore, impairment of GLSR and GLSRe had superior diagnostic accuracy than the quantitative assessment of WMA in the detection of prior MI. These results demonstrate that prior MI may be detected when GLS is impaired when LVEF is preserved, although its ability to detect prior MI in this context is moderate.
Impairment of longitudinal measures of strain in prior MI may relate to myocardial fiber arrangement within the left ventricle. Subendocardial myofibers contribute predominantly to contraction in the longitudinal plane with subepicardial fibers providing a lesser contribution. Conversely, both circumferential and radial motion of the myocardium are generated by fibers predominantly located in the midwall [9]. Longitudinal function is particularly vulnerable to any disease process affecting the subendocardium. It is therefore possible that in limited subendocardial MI, longitudinal myocardial deformation is reduced whereas radial function, which results from contraction of LV midwall fibers, may be relatively preserved. Given that LVEF and systolic wall thickening are predominantly measures of radial contraction, this would explain the findings in this study that minimum SWT, presence of WMA and LVEF were all poor predictors of prior MI in patients with preserved LV function. Delgado et al. demonstrated that although GLS was reduced in patients with HF-REF due to chronic ischemic heart disease, the relationship between LVEF and GLS was only weakly linear (r = 0.62, p = <0.001) [15] which would again potentially support the concept of impaired GLS being largely attributable to loss of subendocardial myocardial fiber contraction.
There was no significant difference in the proportion of patients with WMA (>30% minimum SWT in any LV segments) between the prior MI and control groups. Our finding of WMA in asymptomatic controls was not unexpected and has previously been reported elsewhere in the literature [17, 22].
The importance of LVEF and regional LV systolic function as indicators of prior MI is stressed in international guidelines [1]. Our results demonstrate the potential additive value of longitudinal strain parameters and in particular GLS in looking beyond traditional means of assessing LV systolic function in the detection of prior MI.
Of the longitudinal strain parameters assessed, GLS was moderately useful in terms of specificity and sensitivity with a cut-off value of ≥18% giving a sensitivity of 60% and specificity of 72.5% and was the most useful of the 3 strain values assessed. These findings broadly correlate with those of Nucifora et al. who demonstrated a correlation between reduced GLS measured by echocardiography and the presence of significant coronary artery disease on CT coronary angiography in patients with symptoms suggestive of stable angina with normal LVEF [23]. In that study, the authors proposed a cut off value ≥−17.4% predicting significant coronary artery disease with a sensitivity of 83% and specificity of 77%. It is unclear why our own values for the sensitivity and specificity of GLS in identifying prior MI were lower, though one reason could be that ischemia has a more profound effect on GLS than infarction, possibly due to the influence of LV remodeling in the context of chronic infarction.
GLS has good inter and intra-observer variability when measured by CMR feature tracking as demonstrated in both this and another study [24]. Similar values have also been demonstrated using echocardiography [25]. The findings of this study adds to the growing evidence base supporting the use of GLS in clinical practice. It has shown potential in terms of prognostication and has been demonstrated to be superior to LVEF in predicting morbidity and mortality in patients with IHD [26]. Additionally, it shows promise as a potential screening tool for silent MI [27].
Several studies have demonstrated impairment of GLS, predominantly by speckle tracking echocardiography, in a range of disease states including diabetes [28], heart failure with preserved ejection fraction (HFPEF) [29] and aortic stenosis [30]. In these studies, impairment of longitudinal function was attributed to a direct cardiomyopathic process. However, LGE imaging was not performed in these patients and it is therefore possible that impairment of GLS may have related to unrecognized MI. Thus, future studies of the prognostic importance of GLS should include LGE imaging to exclude MI as the mechanism of impairment of longitudinal function.
Our findings support the potential utility of GLS as a screening tool for identifying prior MI in patients with preserved LV ejection fraction, although when used alone, its ability to correctly identify prior MI in this context is only moderate. Further prospective studies are needed to identify whether combining it with other imaging and/or clinical parameters lead to improvement in its specificity and sensitivity. As GLS has been shown to be impaired independently of LVEF in aortic stenosis [30], hypertrophic cardiomyopathy [31] and HFPEF [29], it must be interpreted with caution where these conditions are present or are suspected.
Limitations of the study
The values for the strain parameters measured in this study were calculated using feature tracking post-processing software. This remains a research application and currently lacks the clinical validation to enable its adoption into routine clinical practice. Nevertheless, both GLS and GLSR can be readily measured using modern echocardiographic speckle tracking [12] which have been validated against CMR strain measurements [32].
CMR tagging has traditionally been considered the reference standard technique for calculation of strain values and was not used in this study. We have elected to use feature tracking preferentially for this study because tagging techniques suffer from both lower temporal resolution and fading of the tag overlay as the cardiac cycle progresses [24]. Furthermore agreement between feature tracking and CMR tagging is excellent [33] and can be easily performed without the need for acquisition of additional sequences.
We have not carried out invasive assessment of coronary anatomy in all patients and it is possible that undiagnosed ischaemia may have contributed to impairment of longitudinal strain parameters. However, performing coronary angiography on patients in whom it is not clinically indicated would not be ethically appropriate.
Finally, there were higher rates of CV risk factors in our prior MI population compared with healthy volunteers, thus it is unclear as to their relative contribution (if any) to the observed decrease in GLS seen in prior MI patients. Nevertherless, in our multivariable analysis, only prior MI was an independent predictor of change in GLS and CV risk factors including hypertension, smoking, hypercholesterolemia and diabetes were not.
Conclusion
The strain parameters GLS, GLSR and GLSRe are reduced in patients with prior MI in the context of normal LVEF. A normal LVEF and lack of WMA is insufficient to exclude prior MI. Prior MI may be suspected when impaired GLS, GLSR or GLSRe are detected by non-invasive imaging.
References
Thygesen K, Alpert JS, Jaffe AS et al (2012) Third universal definition of myocardial infarction. Eur Heart J 33:2551–2567. doi:10.1093/eurheartj/ehs184
Davis TME, Fortun P, Mulder J et al (2004) Silent myocardial infarction and its prognosis in a community-based cohort of Type 2 diabetic patients: the Fremantle Diabetes Study. Diabetologia 47:395–399. doi:10.1007/s00125-004-1344-4
Sheifer SE, Gersh BJ, Yanez ND 3rd et al (2000) Prevalence, predisposing factors, and prognosis of clinically unrecognized myocardial infarction in the elderly. J Am Coll Cardiol 35:119–126
Hendel RC, Patel MR, Kramer CM et al (2006) ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging. J Am Coll Cardiol 48:1475–1497
Motwani M, Kidambi A, Greenwood JP et al (2014) Advances in cardiovascular magnetic resonance in ischaemic heart disease and non-ischaemic cardiomyopathies. Heart 100:1722–1733. doi:10.1136/heartjnl-2013-304680
EB S, JJ C, Sigurdsson S, al et (2012) Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA 308:890–896
Kwong RY, Chan AK, Brown KA et al (2006) Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. doi:10.1161/CIRCULATIONAHA.105.570648
Perk J, De Backer G, Gohlke H et al (2012) European guidelines on cardiovascular disease prevention in clinical practice (version 2012). Eur Heart J 33:1635–1701. doi:10.1093/eurheartj/ehs092
Sengupta PP, Krishnamoorthy VK, Korinek J et al (2007) Left ventricular form and function revisited: applied translational science to cardiovascular ultrasound imaging. J Am Soc Echocardiogr 20:539–551. doi:10.1016/j.echo.2006.10.013
Buckberg G, Hoffman JIE, Mahajan A et al (2008) Cardiac mechanics revisited: the relationship of cardiac architecture to ventricular function. Circulation 118:2571–2587. doi:10.1161/CIRCULATIONAHA.107.754424
Garg P, Kidambi A, Foley JRJ et al (2016) Ventricular longitudinal function is associated with microvascular obstruction and intramyocardial haemorrhage. Open Heart 3:e000337. doi:10.1136/openhrt-2015-000337
Blessberger H, Binder T (2010) Non-invasive imaging: two dimensional speckle tracking echocardiography: basic principles. Heart 96:716–722. doi:10.1136/hrt.2007.141002
Smiseth OA, Torp H, Opdahl A et al (2015) Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J 37:1196–1207. doi:10.1093/eurheartj/ehv529
Amundsen BH, Crosby J, Steen PA et al (2009) Regional myocardial long-axis strain and strain rate measured by different tissue Doppler and speckle tracking echocardiography methods: a comparison with tagged magnetic resonance imaging. Eur J Echocardiogr 10:229–237. doi:10.1093/ejechocard/jen201
Delgado V, Mollema SA, Ypenburg C et al (2008) Relation between global left ventricular longitudinal strain assessed with novel automated function imaging and biplane left ventricular ejection fraction in patients with coronary artery disease. J Am Soc Echocardiogr 21:1244–1250. doi:10.1016/j.echo.2008.08.010
Ersbøll M, Valeur N, Mogensen UM et al (2013) Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol 61:2365–2373. doi:10.1016/j.jacc.2013.02.061
Yan RT, Bluemke D, Gomes A et al (2011) Regional left ventricular myocardial dysfunction as a predictor of incident cardiovascular events: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol 57:1735–1744. doi:10.1016/j.jacc.2010.10.060
Nowosielski M, Schocke M, Mayr A et al (2009) Comparison of wall thickening and ejection fraction by cardiovascular magnetic resonance and echocardiography in acute myocardial infarction. J Cardiovasc Magn Reson. doi:10.1186/1532-429X-11-22
Flett AS, Hasleton J, Cook C et al (2011) Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC Cardiovasc Imaging 4:150–156. doi:10.1016/j.jcmg.2010.11.015
Pedrizzetti G, Claus P, Kilner PJ, Nagel E (2016) Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J Cardiovasc Magn Reson. doi:10.1186/s12968-016-0269-7
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Cicala S, De Simone G, Roman MJ et al (2007) Prevalence and prognostic significance of wall-motion abnormalities in adults without clinically recognized cardiovascular disease: the strong heart study. Circulation 116:143–150. doi:10.1161/CIRCULATIONAHA.106.652149
Nucifora G, Schuijf JD, Delgado V et al (2010) Incremental value of subclinical left ventricular systolic dysfunction for the identification of patients with obstructive coronary artery disease. Am Heart J 159:148–157. doi:10.1016/j.ahj.2009.10.030
Augustine D, Lewandowski a J, Lazdam M et al (2013) Global and regional left ventricular myocardial deformation measures by magnetic resonance feature tracking in healthy volunteers: comparison with tagging and relevance of gender. J Cardiovasc Magn Reson 15:8. doi:10.1186/1532-429X-15-8
Mignot A, Donal E, Zaroui A et al (2010) Global longitudinal strain as a major predictor of cardiac events in patients with depressed left ventricular function: a multicenter study. J Am Soc Echocardiogr 23:1019–1024. doi:10.1016/j.echo.2010.07.019
Antoni ML, Mollema SA, Delgado V et al (2010) Prognostic importance of strain and strain rate after acute myocardial infarction. Eur Heart J 31:1640–1647. doi:10.1093/eurheartj/ehq105
Swoboda PP, McDiarmid AK, Erhayiem B et al (2016) A novel and practical screening tool for the detection of silent myocardial infarction in patients with type 2 diabetes. J Clin Endocrinol Metab. doi:10.1210/jc.2016-1318
Holland DJ, Marwick TH, Haluska BA et al (2015) Subclinical LV dysfunction and 10-year outcomes in type 2 diabetes mellitus. Heart 101:1061–1066. doi:10.1136/heartjnl-2014-307391
Carluccio E, Biagioli P, Alunni G et al (2011) Advantages of deformation indices over systolic velocities in assessment of longitudinal systolic function in patients with heart failure and normal ejection fraction. Eur J Heart Fail 13:292–302. doi:10.1093/eurjhf/hfq203
Ng ACT, Delgado V, Bertini M et al (2011) Alterations in multidirectional myocardial functions in patients with aortic stenosis and preserved ejection fraction: a two-dimensional speckle tracking analysis. Eur Heart J 32:1542–1550. doi:10.1093/eurheartj/ehr084
Reant P, Mirabel M, Lloyd G et al (2016) Global longitudinal strain is associated with heart failure outcomes in hypertrophic cardiomyopathy. Heart 102:741–747. doi:10.1136/heartjnl-2015-308576
Amundsen BH, Helle-Valle T, Edvardsen T et al (2006) Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol 47:789–793. doi:10.1016/j.jacc.2005.10.040
Hor KN, Gottliebson WM, Carson C et al (2010) Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. JACC Cardiovasc Imaging 3:144–151. doi:10.1016/j.jcmg.2009.11.006
Funding
GF is funded by a National Institute for Healthcare Research Grant (Number 11/117/27). SP is funded by a British Heart Foundation fellowship (FS 10/62/2840).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Disclosures
The authors have nothing to disclose.
Ethical approval
The study was conducted in accordance with the declaration of Helsinki and ethical standards of the institutional research committee.
Informed consent
All patients included in the study gave informed consent.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Fent, G.J., Garg, P., Foley, J.R.J. et al. The utility of global longitudinal strain in the identification of prior myocardial infarction in patients with preserved left ventricular ejection fraction. Int J Cardiovasc Imaging 33, 1561–1569 (2017). https://doi.org/10.1007/s10554-017-1138-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-017-1138-7