Abstract
Purpose
Frailty is assessed when making treatment decisions among older women with breast cancer (BC), which in turn impacts survival. We evaluated associations between pre-diagnosis frailty and risks of BC-specific and all-cause mortality in older women.
Methods
We conducted a retrospective cohort study of Medicare beneficiaries ages ≥ 65 years with stage I–III BC using the Surveillance, Epidemiology and End Results-Medicare Health Outcome Survey Data Resource. Frailty was measured using the deficit-accumulation frailty index, categorized as robust, pre-frail, or frail, at baseline and during follow-up. Fine and Gray competing risk and Cox proportional hazards models were used to estimate subdistribution hazard ratios (SHR) and hazard ratios (HR) with 95% confidence intervals (CI) for BC-specific and all-cause mortality, respectively.
Results
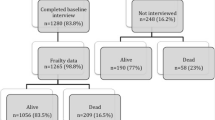
Among 2411 women with a median age of 75 years at BC diagnosis, 49.5% were categorized as robust, 29.4% were pre-frail and 21.1% were frail. Fewer frail women compared to robust women received breast-conserving surgery (52.8% vs. 61.5%, frail vs. robust, respectively) and radiation (43.5% vs. 51.8%). In multivariable analyses, degree of frailty was not associated with BC-specific mortality (frail vs robust SHR 1.47, 95% CI 0.97–2.24). However, frail women with BC had higher risks of all-cause mortality compared to robust women with BC (HR 2.32, 95% CI 1.84–2.92).
Conclusion
Among a cohort of older women with BC, higher degrees of frailty were associated with higher risk of all-cause mortality, but not BC-specific mortality. Future study should examine if preventing progression of frailty may improve all-cause mortality.



Similar content being viewed by others
Data availability
The authors have full control of all primary data. The data that support the findings of this study are available from the SEER-MHOS data resource. Restrictions apply to the availability of these data, which were used under license of this study.
Abbreviations
- BC:
-
Breast cancer
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- DAFI:
-
Deficit-accumulation frailty index
- HR:
-
Hazard ratios
- HRQOL:
-
Health-related quality of life
- IQR:
-
Interquartile ranges
- MHOS:
-
Medicare Health Outcomes Survey
- PH:
-
Proportional hazards
- SEER:
-
Surveillance, Epidemiology and End Results
- SHR:
-
Subdistribution hazard ratios
- US:
-
United States
References
DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Sauer AG et al (2019) Breast cancer statistics, 2019. CA A Cancer J Clin 69:438–451
Ban KA, Godellas CV (2014) Epidemiology of breast cancer. Surg Oncol Clin N Am 23:409–422
Howlader N, Noone A, Krapcho M, Miller D, Brest A, Yu M, et al. (2019) SEER cancer statistics review, 1975–2016 [Internet]. National Cancer Institute. https://seer.cancer.gov/csr/1975_2016/. Accessed 11 Mar 2020
Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K (2013) Frailty in elderly people. Lancet 381:752–762
Torpy JM, Lynm C, Glass RM (2006) Frailty in older adults. JAMA 296:2280–2280
Xue Q-L (2011) The frailty syndrome: definition and natural history. Clin Geriatr Med 27:1–15
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J et al (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A 56:M146–M157
Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS (2018) Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Health 3:e323–e332
Mitnitski AB, Mogilner AJ, MacKnight C, Rockwood K (2002) The accumulation of deficits with age and possible invariants of aging. Sci World J 2:1816–1822
Rockwood K, Mitnitski A (2007) Frailty in relation to the accumulation of deficits. J Gerontol A 62:722–727
de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JSM, Olde Rikkert MGM, Nijhuis-van der Sanden MWG (2011) Outcome instruments to measure frailty: a systematic review. Ageing Res Rev 10:104–114
Chang S-F, Lin P-L (2015) Frail phenotype and mortality prediction: a systematic review and meta-analysis of prospective cohort studies. Int J Nurs Stud 52:1362–1374
Handforth C, Clegg A, Young C, Simpkins S, Seymour MT, Selby PJ et al (2015) The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol 26:1091–1101
Kane RL, Shamliyan T, Talley K, Pacala J (2012) The association between geriatric syndromes and survival. J Am Geriatr Soc 60:896–904
Kojima G, Iliffe S, Walters K (2018) Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age Ageing 47:193–200
Shamliyan T, Talley KMC, Ramakrishnan R, Kane RL (2013) Association of frailty with survival: a systematic literature review. Ageing Res Rev 12:719–736
Clough-Gorr KM, Thwin SS, Stuck AE, Silliman RA (2012) Examining five- and ten-year survival in older women with breast cancer using cancer-specific geriatric assessment. Eur J Cancer 48:805–812
Mandelblatt JS, Cai L, Luta G, Kimmick G, Clapp J, Isaacs C et al (2017) Frailty and long-term mortality of older breast cancer patients: CALGB 369901 (Alliance). Breast Cancer Res Treat 164:107–117
Nechuta S, Lu W, Zheng Y, Cai H, Bao P-P, Gu K et al (2013) Comorbidities and breast cancer survival: a report from the Shanghai breast cancer survival study. Breast Cancer Res Treat 139:227–235
Wu AH, Kurian AW, Kwan ML, John EM, Lu Y, Keegan THM et al (2015) Diabetes and other comorbidities in breast cancer survival by race/ethnicity: the California breast cancer survivorship consortium (CBCSC). Cancer Epidemiol Biomarkers Prev 24:361–368
Yancik R, Wesley MN, Ries LAG, Havlik RJ, Edwards BK, Yates JW (2001) Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA 285:885–892
Ambs A, Warren JL, Bellizzi KM, Topor M, Haffer SC, Clauser SB (2008) Overview of the SEER–medicare health outcomes survey linked dataset. Health Care Financ Rev 29:5–21
About the SEER Program [Internet]. SEER. https://seer.cancer.gov/about/overview.html. Accessed 12 Feb 2020
Hays RD, Sherbourne CD, Mazel RM (1993) The RAND 36-item health survey 1.0. Health Econ 2:217–227
Jones N, Jones SL, Miller NA (2004) The medicare health outcomes survey program: overview, context, and near-term prospects. Health Qual Life Outcomes 2:33
Mian HS, Wildes TM, Fiala MA (2018) Development of a medicare health outcomes survey deficit-accumulation frailty index and its application to older patients with newly diagnosed multiple myeloma. JCO Clin Cancer Inform [Internet]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6289185/. Accessed 12 Feb 2020
Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K (2002) Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr 2:1
Cohen HJ, Smith D, Sun C-L, Tew W, Mohile SG, Owusu C et al (2016) Frailty as determined by a comprehensive geriatric assessment-derived deficit-accumulation index in older patients with cancer who receive chemotherapy. Cancer 122:3865–3872
Peña FG, Theou O, Wallace L, Brothers TD, Gill TM, Gahbauer EA et al (2014) Comparison of alternate scoring of variables on the performance of the frailty index. BMC Geriatr 14:25
Song X, Mitnitski A, Rockwood K (2010) Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc 58:681–687
Theou O, Brothers TD, Mitnitski A, Rockwood K (2013) Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc 61:1537–1551
Theou O, Brothers TD, Peña FG, Mitnitski A, Rockwood K (2014) Identifying common characteristics of frailty across seven scales. J Am Geriatr Soc 62:901–906
Fine JP, Gray RJ (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496–509
Gray RJ (1988) A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141–1154
Nelson W (1972) Theory and applications of hazard plotting for censored failure data. Technometrics 14:945–966
Aalen O (1978) Nonparametric inference for a family of counting processes. Ann Stat 6:701–726
Williams GR, Deal AM, Sanoff HK, Nyrop KA, Guerard EJ, Pergolotti M et al (2019) Frailty and health-related quality of life in older women with breast cancer. Support Care Cancer 27:2693–2698
Jauhari Y, Gannon MR, Dodwell D, Horgan K, Tsang C, Clements K et al (2020) Addressing frailty in patients with breast cancer: a review of the literature. Eur J Surg Oncol 46:24–32
Kirkhus L, Šaltytė Benth J, Rostoft S, Grønberg BH, Hjermstad MJ, Selbæk G et al (2017) Geriatric assessment is superior to oncologists’ clinical judgement in identifying frailty. Br J Cancer 117:470–477
Stover AM, Mayer DK, Muss H, Wheeler SB, Lyons JC, Reeve BB (2014) Quality of life changes during the pre- to postdiagnosis period and treatment-related recovery time in older women with breast cancer. Cancer 120:1881–1889
Pinquart M, Duberstein PR (2010) Depression and cancer mortality: a meta-analysis. Psychol Med Camb Univ Press 40:1797–1810
Satin JR, Linden W, Phillips MJ (2009) Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer 115:5349–5361
Wang YH, Li JQ, Shi JF, Que JY, Liu JJ, Lappin JM et al (2020) Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta-analysis of cohort studies. Mol Psychiatry 25(7):1487–1499. https://doi.org/10.1038/s41380-019-0595-x
Soysal P, Veronese N, Thompson T, Kahl KG, Fernandes BS, Prina AM et al (2017) Relationship between depression and frailty in older adults: a systematic review and meta-analysis. Ageing Res Rev 36:78–87
Andersen BL, DeRubeis RJ, Berman BS, Gruman J, Champion VL, Massie MJ et al (2014) Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American society of clinical oncology guideline adaptation. J Clin Oncol 32:1605–1619
Fayanju OM, Ren Y, Thomas SM, Greenup RA, Hyslop T, Hwang ES, et al. (2020) A case-control study examining disparities in clinical trial participation among breast surgical oncology patients. JNCI Cancer Spectr [Internet]. https://academic.oup.com/jncics/article/4/2/pkz103/5678798. Accessed 30 Jun 2020
Lemieux J, Forget G, Brochu O, Provencher L, Cantin G, Desbiens C et al (2014) Evaluation of eligibility and recruitment in breast cancer clinical trials. Breast 23:385–392
Sateren WB, Trimble EL, Abrams J, Brawley O, Breen N, Ford L et al (2002) How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol 20:2109–2117
DuGoff E, Buckingham W, Kind AJH, Chao S, Anderson G (2019) Targeting high-need beneficiaries in medicare advantage: opportunities to address medical and social needs [Internet]. The commonwealth fund. https://www.commonwealthfund.org/publications/issue-briefs/2019/feb/targeting-high-need-beneficiaries-medicare-advantage. Accessed 1 Feb 2019
Teigland C, Pulungan Z, Shah T, Schneider E, Bishop S (2020) As it grows, medicare advantage is enrolling more low-income and medically complex beneficiaries: recent trends in beneficiary clinical characteristics, health care utilization, and spending [Internet]. The commonwealth fund. https://www.commonwealthfund.org/publications/issue-briefs/2020/may/medicare-advantage-enrolling-low-income-medically-complex. Accessed 13 May 2020
Ritchie CS, Kvale E, Fisch MJ (2011) Multimorbidity: an issue of growing importance for oncologists. J Oncol Pract 7:371–374
Shrank WH, Patrick AR, Alan BM (2011) Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med 26:546–550
Chan L, Doctor JN, MacLehose RF, Lawson H, Rosenblatt RA, Baldwin LM et al (1999) Do medicare patients with disabilities receive preventive services? A population-based study. Arch Phys Med Rehabil 80:642–646
Wei W, Findley PA, Sambamoorthi U (2006) Disability and receipt of clinical preventive services among women. Women’s Health Issues 16:286–296
Wirtz HS, Calip GS, Buist DSM, Gralow JR, Barlow WE, Gray S et al (2017) Evidence for detection bias by medication use in a cohort study of breast cancer survivors. Am J Epidemiol 185:661–672
Keating NL, Landrum MB, Guadagnoli E, Winer EP, Ayanian JZ (2006) Factors related to underuse of surveillance mammography among breast cancer survivors. J Clin Oncol 24:85–94
Glynn RJ, Schneeweiss S, Wang PS, Levin R, Avorn J (2006) Selective prescribing led to overestimation of the benefits of lipid-lowering drugs. J Clin Epidemiol 59:819–828
Renoux C, Dell’Aniello S, Brenner B, Suissa S (2017) Bias from depletion of susceptibles: the example of hormone replacement therapy and the risk of venous thromboembolism. Pharmacoepidemiol Drug Saf 26:554–560
Acknowledgements
This study used date from the Surveillance, Epidemiology, and End Results (SEER)—Medicare Health Outcomes Survey (MHOS) linked data resource. The authors acknowledge the effects of the National Cancer Institute; the Centers for Medicare and Medicaid Services; MHOS; Information Management Services, Inc; and the SEER Program tumor registries in the creation of the SEER-MHOS database. The National Cancer Institute provided suggested edits and approval of the manuscript before final journal submission.
Funding
Research reported in this publication was supported, in part, by the National Institutes of Health’s National Cancer Institute, Grant Numbers U54CA202995, U54CA202997, and U54CA203000. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, design, data collection, and analyses. The first draft of the manuscript was written by CHY, and all authors reviewed and commented on previous versions of the manuscript. All authors read and provided approval of the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
At the time of submission, Gregory S. Calip reports employment with Flatiron Health, Inc, which is an independent subsidiary of the Roche Group and has received research funding from Pfizer, Inc. awarded to the University of Illinois at Chicago for work unrelated to this study. Chandler Coleman reports current employment with AbbVie, Inc. at the time of submission. All other authors have no conflict of interest to declare.
Ethical approval
The data used in the study were de-identified and compliant with the Health Insurance Portability and Accountability Act (HIPAA). This study was determined to be exempt by the Institutional Review Board of the University of Illinois at Chicago.
Informed consent
The Institutional Review Board of the University of Illinois at Chicago determined this study to be exempt from obtaining informed consent from individual participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yan, C.H., Coleman, C., Nabulsi, N.A. et al. Associations between frailty and cancer-specific mortality among older women with breast cancer. Breast Cancer Res Treat 189, 769–779 (2021). https://doi.org/10.1007/s10549-021-06323-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06323-3