Abstract
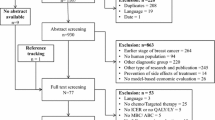
The economic evaluation (EE) of health care products has become a necessity. Their quality must be high in order to trust the results and make informed decisions. While cost–utility analyses (CUAs) should be preferred to cost–effectiveness analyses in the oncology area, the quality of breast cancer (BC)-related CUA has been given little attention so far. Thus, firstly, a systematic review of published CUA related to drug therapies for BC, gene expression profiling, and HER2 status testing was performed. Secondly, the quality of selected CUA was assessed and the factors associated with a high-quality CUA identified. The systematic literature search was conducted in PubMed, MEDLINE/EMBASE, and Cochrane to identify published CUA between 2000 and 2014. After screening and data extraction, the quality of each selected CUA was assessed by two independent reviewers, using the checklist proposed by Drummond et al. The analysis of factors associated with a high-quality CUA (defined as a Drummond score ≥7) was performed using a two-step approach. Our systematic review was based on 140 CUAs and showed a wide variety of methodological approaches, including differences in the perspective adopted, the time horizon, measurement of cost and effectiveness, and more specially health-state utility values (HSUVs). The median Drummond score was 7 [range 3–10]. Only one in two of the CUA (n = 74) had a Drummond score ≥7, synonymous of “high quality.” The statistically significant predictors of a high-quality CUA were article with “gene expression profiling” topic (p = 0.001), consulting or pharmaceutical company as main location of first author (p = 0.004), and articles with both incremental cost–utility ratio and incremental cost–effectiveness ratio as outcomes of EE (p = 0.02). Our systematic review identified only 140 CUAs published over the past 15 years with one in two of high quality. It showed a wide variety of methodological approaches, especially focused on HSUVs. A critical appraisal of utility values is necessary to better understand one of the main difficulties encountered by authors and propose areas for improvement to increase the quality of CUA. Since the last 5 years, there is a tendency toward an improvement in the quality of these studies, probably coupled with economic context, a better and widely spreading of recommendations and thus appropriation by medical practitioners. That being said, there is an urgent need for mandatory use of European and international recommendations to ensure quality of such approaches and to allow easy comparison.



Similar content being viewed by others
References
Wolff AC, Hammond MEH, Hicks DG et al (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31:3997–4013. doi:10.1200/JCO.2013.50.9984
Giordano SH, Temin S, Kirshner JJ et al (2014) Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 32:2078–2099. doi:10.1200/JCO.2013.54.0948
Burstein HJ, Temin S, Anderson H et al (2014) Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol 32:2255–2269. doi:10.1200/JCO.2013.54.2258
Partridge AH, Rumble RB, Carey LA et al (2014) Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) advanced breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 32:3307–3329. doi:10.1200/JCO.2014.56.7479
Van Poznak C, Somerfield MR, Bast RC et al (2015) Use of biomarkers to guide decisions on systemic therapy for women with metastatic breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 33:2695–2704. doi:10.1200/JCO.2015.61.1459
Paik S, Shak S, Tang G et al (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351:2817–2826. doi:10.1056/NEJMoa041588
Paik S, Tang G, Shak S et al (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24:3726–3734. doi:10.1200/JCO.2005.04.7985
Albain KS, Barlow WE, Shak S et al (2010) Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 11:55–65. doi:10.1016/S1470-2045(09)70314-6
Lidgren M, Wilking N, Jönsson B, Rehnberg C (2007) Health related quality of life in different states of breast cancer. Qual Life Res 16:1073–1081. doi:10.1007/s11136-007-9202-8
Luengo-Fernandez R, Leal J, Gray A, Sullivan R (2013) Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol 14:1165–1174. doi:10.1016/S1470-2045(13)70442-X
Projet de Loi de de Financement de la Sécurtié Sociale 2016. http://www.securite-sociale.fr/LFSS-2016. Accessed 24 March 2016
Situation de la chimiothérapie des cancers - Année 2014. http://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Situation-de-la-chimiotherapie-des-cancers-Annee-2014. Accessed 24 March 2016
Greenberg D, Earle C, Fang C-H et al (2010) When is cancer care cost-effective? A systematic overview of cost–utility analyses in oncology. J Natl Cancer Inst 102:82–88. doi:10.1093/jnci/djp472
Frederix GWJ, Severens JL, Hövels AM et al (2013) The cloudy crystal ball of cost–effectiveness studies. Value Health 16:1100–1102. doi:10.1016/j.jval.2013.06.012
Haute Autorité de Santé—choices in methods for economic evaluation—a methodological guide. http://www.has-sante.fr/portail/jcms/r_1499251/en/choices-in-methods-for-economic-evaluation. Accessed 24 March 2016
Husereau D, Drummond M, Petrou S et al (2013) Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 16:231–250. doi:10.1016/j.jval.2013.02.002
Federal reserve system—foreign exchange rates. http://www.federalreserve.gov/releases/g5a/current/default.htm. Accessed 24 March 2016
Adams ME, McCall NT, Gray DT et al (1992) Economic analysis in randomized control trials. Med Care 30:231–243
Drummond MF, Jefferson TO (1996) Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 313:275–283
Gold M, Siegel J, Russell L, Weinstein M (1996) Cost–effectiveness in health and medicine. Oxford University Press, New York
Evers S, Goossens M, de Vet H et al (2005) Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care 21:240–245
Drummond M, Sculpher M, O’Brien B (2005) Methods for the economic evaluation of health care programmes. Oxford University Press, Torrance GW
Hillner BE, Weeks JC, Desch CE, Smith TJ (2000) Pamidronate in prevention of bone complications in metastatic breast cancer: a cost–effectiveness analysis. J Clin Oncol 18:72–79
Dranitsaris G, Leung P, Mather J, Oza A (2000) Cost–utility analysis of second-line hormonal therapy in advanced breast cancer: a comparison of two aromatase inhibitors to megestrol acetate. Anticancer Drugs 11:591–601
Brown RE, Hutton J, Burrell A (2001) Cost effectiveness of treatment options in advanced breast cancer in the UK. Pharmacoeconomics 19:1091–1102
Li N, van Agthoven M, Willemse P, Uyl-de Groot C (2001) A cost–utility analysis comparing second-line chemotherapy schemes in patients with metastatic breast cancer. Anticancer Drugs 12:533–540
Karnon J, Brown J, Adjuvant Breast Cancer (ABC) Steering Committee (2002) Tamoxifen plus chemotherapy versus tamoxifen alone as adjuvant therapies for node-positive postmenopausal women with early breast cancer: a stochastic economic evaluation. Pharmacoeconomics 20:119–137
Martin SC, Gagnon DD, Zhang L et al (2003) Cost–utility analysis of survival with epoetin-alfa versus placebo in stage IV breast cancer. Pharmacoeconomics 21:1153–1169
Dranitsaris G, Verma S, Trudeau M (2003) Cost utility analysis of first-line hormonal therapy in advanced breast cancer: comparison of two aromatase inhibitors to tamoxifen. Am J Clin Oncol 26:289–296. doi:10.1097/01.COC.0000021042.55557.2B
Karnon J, Johnston SRD, Jones T, Glendenning A (2003) A trial-based cost–effectiveness analysis of letrozole followed by tamoxifen versus tamoxifen followed by letrozole for postmenopausal advanced breast cancer. Ann Oncol 14:1629–1633
Simons WR, Jones D, Buzdar A (2003) Cost–effectiveness of anastrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer. Clin Ther 25:2972–2987
Elkin EB, Weinstein MC, Winer EP et al (2004) HER-2 testing and trastuzumab therapy for metastatic breast cancer: a cost–effectiveness analysis. J Clin Oncol 22:854–863. doi:10.1200/JCO.2004.04.158
Marchetti M, Caruggi M, Colombo G (2004) Cost utility and budget impact of third-generation aromatase inhibitors for advanced breast cancer: a literature-based model analysis of costs in the Italian National Health Service. Clin Ther 26:1546–1561. doi:10.1016/j.clinthera.2004.09.014
Hillner BE (2004) Benefit and projected cost–effectiveness of anastrozole versus tamoxifen as initial adjuvant therapy for patients with early-stage estrogen receptor-positive breast cancer. Cancer 101:1311–1322. doi:10.1002/cncr.20492
Naeim A, Keeler EB (2005) Is adjuvant therapy for older patients with node (−) early breast cancer cost-effective? Crit Rev Oncol Hematol 53:81–89. doi:10.1016/j.critrevonc.2004.09.001
Hornberger J, Cosler LE, Lyman GH (2005) Economic analysis of targeting chemotherapy using a 21-gene RT-PCR assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer. Am J Manag Care 11:313–324
Verma S, Maraninchi D, O’Shaughnessy J et al (2005) Capecitabine plus docetaxel combination therapy. Cancer 103:2455–2465. doi:10.1002/cncr.21122
De Cock E, Hutton J, Canney P et al (2005) Cost–effectiveness of oral ibandronate versus IV zoledronic acid or IV pamidronate for bone metastases in patients receiving oral hormonal therapy for breast cancer in the United Kingdom. Clin Ther 27:1295–1310. doi:10.1016/j.clinthera.2005.08.006
Oestreicher N, Ramsey SD, Linden HM et al (2005) Gene expression profiling and breast cancer care: What are the potential benefits and policy implications? Genet Med 7:380–389. doi:10.1097/01.GIM.0000170776.31248.75
Naeim A, Keeler EB (2005) Is adjuvant therapy for older patients with node (+) early breast cancer cost-effective? Breast Cancer Res Treat 94:95–103. doi:10.1007/s10549-004-8267-0
De Cock E, Hutton J, Canney P et al (2005) Cost–effectiveness of oral ibandronate compared with intravenous (i.v.) zoledronic acid or i.v. generic pamidronate in breast cancer patients with metastatic bone disease undergoing i.v. chemotherapy. Support Care Cancer 13:975–986. doi:10.1007/s00520-005-0828-1
Karnon J, Delea T, Johnston SRD et al (2006) Cost effectiveness of extended adjuvant letrozole in postmenopausal women after adjuvant tamoxifen therapy: the UK perspective. Pharmacoeconomics 24:237–250
Moeremans K, Annemans L (2006) Cost–effectiveness of anastrozole compared to tamoxifen in hormone receptor-positive early breast cancer. Analysis based on the ATAC trial. Int J Gynecol Cancer 16(Suppl 2):576–578. doi:10.1111/j.1525-1438.2006.00699.x
Lønning PE (2006) Comparing cost/utility of giving an aromatase inhibitor as monotherapy for 5 years versus sequential administration following 2–3 or 5 years of tamoxifen as adjuvant treatment for postmenopausal breast cancer. Ann Oncol 17:217–225. doi:10.1093/annonc/mdj048
Gil JM, Rubio-Terrés C, Del Castillo A et al (2006) Pharmacoeconomic analysis of adjuvant therapy with exemestane, anastrozole, letrozole or tamoxifen in postmenopausal women with operable and estrogen receptor-positive breast cancer. Clin Transl Oncol 8:339–348
Limwattananon S, Limwattananon C, Maoleekulpairoj S, Soparatanapaisal N (2006) Cost–effectiveness analysis of sequential paclitaxel adjuvant chemotherapy for patients with node positive primary breast cancer. J Med Assoc Thail 89:690–698
Botteman M, Barghout V, Stephens J et al (2006) Cost effectiveness of bisphosphonates in the management of breast cancer patients with bone metastases. Ann Oncol 17:1072–1082. doi:10.1093/annonc/mdl093
Delea TE, Karnon J, Smith RE et al (2006) Cost–effectiveness of extended adjuvant letrozole therapy after 5 years of adjuvant tamoxifen therapy in postmenopausal women with early-stage breast cancer. Am J Manag Care 12:374–386
Rocchi A, Verma S (2006) Anastrozole is cost-effective vs tamoxifen as initial adjuvant therapy in early breast cancer: Canadian perspectives on the ATAC completed-treatment analysis. Support Care Cancer 14:917–927. doi:10.1007/s00520-006-0035-8
Fagnoni P, Limat S, Chaigneau L et al (2006) Clinical and economic impact of epoetin in adjuvant-chemotherapy for breast cancer. Support Care Cancer 14:1030–1037. doi:10.1007/s00520-006-0062-5
Millar JA, Millward MJ (2007) Cost effectiveness of trastuzumab in the adjuvant treatment of early breast cancer: a lifetime model. Pharmacoeconomics 25:429–442
Norum J, Olsen JA, Wist EA, Lønning PE (2007) Trastuzumab in adjuvant breast cancer therapy. A model based cost–effectiveness analysis. Acta Oncol 46:153–164. doi:10.1080/02841860601096841
El Ouagari K, Karnon J, Delea T et al (2007) Cost–effectiveness of letrozole in the extended adjuvant treatment of women with early breast cancer. Breast Cancer Res Treat 101:37–49. doi:10.1007/s10549-006-9262-4
Kurian AW, Thompson RN, Gaw AF et al (2007) A cost–effectiveness analysis of adjuvant trastuzumab regimens in early HER2/neu-positive breast cancer. J Clin Oncol 25:634–641. doi:10.1200/JCO.2006.06.3081
Liberato NL, Marchetti M, Barosi G (2007) Cost effectiveness of adjuvant trastuzumab in human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 25:625–633. doi:10.1200/JCO.2006.06.4220
Skedgel C, Rayson D, Dewar R, Younis T (2007) Cost–utility of adjuvant hormone therapies for breast cancer in post-menopausal women: sequential tamoxifen-exemestane and upfront anastrozole. Breast Cancer Res Treat 101:325–333. doi:10.1007/s10549-006-9299-4
Lyman GH, Cosler LE, Kuderer NM, Hornberger J (2007) Impact of a 21-gene RT-PCR assay on treatment decisions in early-stage breast cancer: an economic analysis based on prognostic and predictive validation studies. Cancer 109:1011–1018. doi:10.1002/cncr.22506
Lundkvist J, Wilking N, Holmberg S, Jönsson L (2007) Cost–effectiveness of exemestane versus tamoxifen as adjuvant therapy for early-stage breast cancer after 2–3 years treatment with tamoxifen in Sweden. Breast Cancer Res Treat 102:289–299. doi:10.1007/s10549-006-9333-6
Delea TE, Karnon J, Sofrygin O et al (2007) Cost–effectiveness of letrozole versus tamoxifen as initial adjuvant therapy in hormone receptor-positive postmenopausal women with early-stage breast cancer. Clin Breast Cancer 7:608–618. doi:10.3816/CBC.2007.n.018
Skedgel C, Rayson D, Dewar R, Younis T (2007) Cost–utility of adjuvant hormone therapies with aromatase inhibitors in post-menopausal women with breast cancer: upfront anastrozole, sequential tamoxifen–exemestane and extended tamoxifen–letrozole. Breast 16:252–261. doi:10.1016/j.breast.2006.12.002
Mansel R, Locker G, Fallowfield L et al (2007) Cost–effectiveness analysis of anastrozole vs tamoxifen in adjuvant therapy for early stage breast cancer in the United Kingdom: the 5-year completed treatment analysis of the ATAC (‘Arimidex’, Tamoxifen alone or in combination) trial. Br J Cancer 97:152–161. doi:10.1038/sj.bjc.6603804
Garrison LP, Lubeck D, Lalla D et al (2007) Cost–effectiveness analysis of trastuzumab in the adjuvant setting for treatment of HER2-positive breast cancer. Cancer 110:489–498. doi:10.1002/cncr.22806
Risebrough NA, Verma S, Trudeau M, Mittmann N (2007) Cost–effectiveness of switching to exemestane versus continued tamoxifen as adjuvant therapy for postmenopausal women with primary breast cancer. Cancer 110:499–508. doi:10.1002/cncr.22824
Thompson D, Taylor DCA, Montoya EL et al (2007) Cost–effectiveness of switching to exemestane after 2 to 3 years of therapy with tamoxifen in postmenopausal women with early-stage breast cancer. Value Health 10:367–376. doi:10.1111/j.1524-4733.2007.00190.x
Locker GY, Mansel R, Cella D et al (2007) Cost–effectiveness analysis of anastrozole versus tamoxifen as primary adjuvant therapy for postmenopausal women with early breast cancer: a US healthcare system perspective. The 5-year completed treatment analysis of the ATAC (‘Arimidex’, Tamoxifen Alone or in Combination) trial. Breast Cancer Res Treat 106:229–238. doi:10.1007/s10549-006-9483-6
Lidgren M, Wilking N, Jönsson B, Rehnberg C (2008) Cost–effectiveness of HER2 testing and trastuzumab therapy for metastatic breast cancer. Acta Oncol 47:1018–1028. doi:10.1080/02841860801901618
Wolowacz SE, Cameron DA, Tate HC, Bagust A (2008) Docetaxel in combination with doxorubicin and cyclophosphamide as adjuvant treatment for early node-positive breast cancer: a cost–effectiveness and cost–utility analysis. J Clin Oncol 26:925–933. doi:10.1200/JCO.2006.10.4190
Lidgren M, Jönsson B, Rehnberg C et al (2008) Cost–effectiveness of HER2 testing and 1-year adjuvant trastuzumab therapy for early breast cancer. Ann Oncol 19:487–495. doi:10.1093/annonc/mdm488
Delea TE, El-Ouagari K, Karnon J, Sofrygin O (2008) Cost–effectiveness of letrozole versus tamoxifen as initial adjuvant therapy in postmenopausal women with hormone-receptor positive early breast cancer from a Canadian perspective. Breast Cancer Res Treat 108:375–387. doi:10.1007/s10549-007-9607-7
Marino P, Roché H, Moatti J-P, PEGASE Group (2008) High-dose chemotherapy for patients with high-risk breast cancer: a clinical and economic assessment using a quality-adjusted survival analysis. Am J Clin Oncol 31:117–124. doi:10.1097/COC.0b013e3181573e83
Karnon J, Delea T, Barghout V (2008) Cost utility analysis of early adjuvant letrozole or anastrozole versus tamoxifen in postmenopausal women with early invasive breast cancer: the UK perspective. Eur J Health Econ 9:171–183. doi:10.1007/s10198-007-0058-1
Younis T, Rayson D, Sellon M, Skedgel C (2008) Adjuvant chemotherapy for breast cancer: a cost–utility analysis of FEC-D vs. FEC 100. Breast Cancer Res Treat 111:261–267. doi:10.1007/s10549-007-9770-x
Kondo M, Hoshi SL, Ishiguro H et al (2008) Economic evaluation of 21-gene reverse transcriptase-polymerase chain reaction assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer in Japan. Breast Cancer Res Treat 112:175–187. doi:10.1007/s10549-007-9842-y
Cameron DA, Camidge DR, Oyee J, Hirsch M (2008) Economic evaluation of fulvestrant as an extra step in the treatment sequence for ER-positive advanced breast cancer. Br J Cancer 99:1984–1990. doi:10.1038/sj.bjc.6604790
Benedict A, Cameron DA, Corson H, Jones SE (2009) An economic evaluation of docetaxel and paclitaxel regimens in metastatic breast cancer in the UK. Pharmacoeconomics 27:847–859. doi:10.2165/10899510-000000000-00000
Borget I, Di Palma M, Leonard R (2009) Pegfilgrastim—a health economic model to assess overall cost–effectiveness. EJHP Pract 15:58–61
Braun S, Mittendorf T, Menschik T et al (2009) Cost effectiveness of exemestane versus tamoxifen in post-menopausal women with early breast cancer in Germany. Breast Care (Basel) 4:389–396. doi:10.1159/000255840
Liu Z, Doan QV, Malin J, Leonard R (2009) The economic value of primary prophylaxis using pegfilgrastim compared with filgrastim in patients with breast cancer in the UK. Appl Health Econ Health Policy 7:193–205. doi:10.2165/11314740-000000000-00000
Liubao P, Xiaomin W, Chongqing T et al (2009) Cost–effectiveness analysis of adjuvant therapy for operable breast cancer from a Chinese perspective: doxorubicin plus cyclophosphamide versus docetaxel plus cyclophosphamide. Pharmacoeconomics 27:873–886. doi:10.2165/11314750-000000000-00000
Martín-Jiménez M, Rodríguez-Lescure A, Ruiz-Borrego M et al (2009) Cost–effectiveness analysis of docetaxel (Taxotere) vs. 5-fluorouracil in combined therapy in the initial phases of breast cancer. Clin Transl Oncol 11:41–47
Maniadakis N, Dafni U, Fragoulakis V et al (2009) Economic evaluation of taxane-based first-line chemotherapy in the treatment of patients with metastatic breast cancer in Greece: an analysis alongside a multicenter, randomized phase III clinical trial. Ann Oncol 20:278–285. doi:10.1093/annonc/mdn634
Le QA, Hay JW (2009) Cost–effectiveness analysis of lapatinib in HER-2-positive advanced breast cancer. Cancer 115:489–498. doi:10.1002/cncr.24033
Au H-J, Golmohammadi K, Younis T et al (2009) Cost–effectiveness analysis of adjuvant docetaxel, doxorubicin, and cyclophosphamide (TAC) for node-positive breast cancer: modeling the downstream effects. Breast Cancer Res Treat 114:579–587. doi:10.1007/s10549-008-0034-1
Danova M, Chiroli S, Rosti G, Doan QV (2009) Cost–effectiveness of pegfilgrastim versus six days of filgrastim for preventing febrile neutropenia in breast cancer patients. Tumori 95:219–226
Lee SG, Jee YG, Chung HC et al (2009) Cost–effectiveness analysis of adjuvant therapy for node positive breast cancer in Korea: docetaxel, doxorubicin and cyclophosphamide (TAC) versus fluorouracil, doxorubicin and cyclophosphamide (FAC). Breast Cancer Res Treat 114:589–595. doi:10.1007/s10549-008-0035-0
Ramsey SD, Liu Z, Boer R et al (2009) Cost–effectiveness of primary versus secondary prophylaxis with pegfilgrastim in women with early-stage breast cancer receiving chemotherapy. Value Health 12:217–225. doi:10.1111/j.1524-4733.2008.00434.x
Van Vlaenderen I, Canon JL, Cocquyt V et al (2009) Trastuzumab treatment of early stage breast cancer is cost-effective from the perspective of the Belgian health care authorities. Acta Clin Belg 64:100–112. doi:10.1179/acb.2009.019
Dedes KJ, Matter-Walstra K, Schwenkglenks M et al (2009) Bevacizumab in combination with paclitaxel for HER-2 negative metastatic breast cancer: an economic evaluation. Eur J Cancer 45:1397–1406. doi:10.1016/j.ejca.2008.12.016
Lyman GH, Lalla A, Barron RL, Dubois RW (2009) Cost–effectiveness of pegfilgrastim versus filgrastim primary prophylaxis in women with early-stage breast cancer receiving chemotherapy in the United States. Clin Ther 31:1092–1104. doi:10.1016/j.clinthera.2009.05.003
Reed SD, Li Y, Anstrom KJ, Schulman KA (2009) Cost effectiveness of ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol 27:2185–2191. doi:10.1200/JCO.2008.19.6352
Dranitsaris G, Cottrell W, Spirovski B, Hopkins S (2009) Economic analysis of albumin-bound paclitaxel for the treatment of metastatic breast cancer. J Oncol Pharm Pract 15:67–78. doi:10.1177/1078155208098584
Skedgel C, Rayson D, Younis T (2009) The cost–utility of sequential adjuvant trastuzumab in women with Her2/Neu-positive breast cancer: an analysis based on updated results from the HERA Trial. Value Health 12:641–648. doi:10.1111/j.1524-4733.2009.00511.x
Lux MP, Hartmann M, Jackisch C et al (2009) Cost–utility analysis for advanced breast cancer therapy in Germany: results of the fulvestrant sequencing model. Breast Cancer Res Treat 117:305–317. doi:10.1007/s10549-008-0294-9
Chen W, Jiang Z, Shao Z et al (2009) An economic evaluation of adjuvant trastuzumab therapy in HER2-positive early breast cancer. Value Health 12(Suppl 3):S82–S84. doi:10.1111/j.1524-4733.2009.00634.x
Chen E, Tong KB, Malin JL (2010) Cost–effectiveness of 70-gene MammaPrint signature in node-negative breast cancer. Am J Manag Care 16:e333–e342
Lee H-J, Lee T-J, Yang B-M, Min J (2010) Cost–effectiveness analysis of adjuvant hormonal treatments for women with postmenopausal hormone-receptor positive early breast cancer in the Korean context. J Breast Cancer 13:286–298. doi:10.4048/jbc.2010.13.3.286
Lux MP, Reichelt C, Wallwiener D et al (2010) Results of the Zometa cost–utility model for the German healthcare system based on the results of the ABCSG-12 study. Onkologie 33:360–368. doi:10.1159/000315699
Lux MP, Wöckel A, Benedict A et al (2010) Cost–effectiveness analysis of anastrozole versus tamoxifen in adjuvant therapy for early-stage breast cancer—a health-economic analysis based on the 100-month analysis of the ATAC trial and the German health system. Onkologie 33:155–166. doi:10.1159/000286233
Tsoi DT, Inoue M, Kelly CM et al (2010) Cost–effectiveness analysis of recurrence score-guided treatment using a 21-gene assay in early breast cancer. Oncologist 15:457–465. doi:10.1634/theoncologist.2009-0275
Ishiguro H, Kondo M, Hoshi S-L et al (2010) Economic evaluation of intensive chemotherapy with prophylactic granulocyte colony-stimulating factor for patients with high-risk early breast cancer in Japan. Clin Ther 32:311–326. doi:10.1016/j.clinthera.2010.01.029
Mittmann N, Verma S, Koo M et al (2010) Cost effectiveness of TAC versus FAC in adjuvant treatment of node-positive breast cancer. Curr Oncol 17:7–16
Retèl VP, Joore MA, Knauer M et al (2010) Cost–effectiveness of the 70-gene signature versus St. Gallen guidelines and Adjuvant Online for early breast cancer. Eur J Cancer 46:1382–1391. doi:10.1016/j.ejca.2010.02.035
Klang SH, Hammerman A, Liebermann N et al (2010) Economic implications of 21-gene breast cancer risk assay from the perspective of an Israeli-managed health-care organization. Value Health 13:381–387. doi:10.1111/j.1524-4733.2010.00724.x
Logman JFS, Heeg BMS, Botteman MF et al (2010) Economic evaluation of zoledronic acid for the prevention of osteoporotic fractures in postmenopausal women with early-stage breast cancer receiving aromatase inhibitors in the UK. Ann Oncol 21:1529–1536. doi:10.1093/annonc/mdp560
Marino P, Siani C, Roché H et al (2010) Cost–effectiveness of adjuvant docetaxel for node-positive breast cancer patients: results of the PACS 01 economic study. Ann Oncol 21:1448–1454. doi:10.1093/annonc/mdp561
Delea TE, Taneja C, Sofrygin O et al (2010) Cost–effectiveness of zoledronic acid plus endocrine therapy in premenopausal women with hormone-responsive early breast cancer. Clin Breast Cancer 10:267–274. doi:10.3816/CBC.2010.n.034
Frías C, Cortés J, Seguí MÁ et al (2010) Cost–effectiveness analyses of docetaxel versus paclitaxel once weekly in patients with metastatic breast cancer in progression following anthracycline chemotherapy, in Spain. Clin Transl Oncol 12:692–700. doi:10.1007/s12094-010-0579-4
Lipsitz M, Delea TE, Guo A (2010) Cost effectiveness of letrozole versus anastrozole in postmenopausal women with HR+ early-stage breast cancer. Curr Med Res Opin 26:2315–2328. doi:10.1185/03007995.2010.510784
Blank PR, Schwenkglenks M, Moch H, Szucs TD (2010) Human epidermal growth factor receptor 2 expression in early breast cancer patients: a Swiss cost–effectiveness analysis of different predictive assay strategies. Breast Cancer Res Treat 124:497–507. doi:10.1007/s10549-010-0862-7
Matter-Walstra KW, Dedes KJ, Schwenkglenks M et al (2010) Trastuzumab beyond progression: a cost–utility analysis. Ann Oncol 21:2161–2168. doi:10.1093/annonc/mdq250
Avritscher EBC, Shih Y-CT, Sun CC et al (2010) Cost–utility analysis of palonosetron-based therapy in preventing emesis among breast cancer patients. J Support Oncol 8:242–251
Thompson MF, Chen L, Tangirala M et al (2011) Cost–effectiveness of docetaxel–cyclophosphamide versus doxorubicin–cyclophosphamide for. Breast Cancer 3:276–283
Vanderlaan BF, Broder MS, Chang EY et al (2011) Cost–effectiveness of 21-gene assay in node-positive, early-stage breast cancer. Am J Manag Care 17:455–464
Bernard LM, Verma S, Thompson MF et al (2011) A Canadian economic analysis of U.S. Oncology Adjuvant Trial 9735. Curr Oncol 18:67–75
Purmonen TT, Pänkäläinen E, Turunen JHO et al (2011) Short-course adjuvant trastuzumab therapy in early stage breast cancer in Finland: cost–effectiveness and value of information analysis based on the 5-year follow-up results of the FinHer Trial. Acta Oncol 50:344–352. doi:10.3109/0284186X.2011.553841
Hall PS, Hulme C, McCabe C et al (2011) Updated cost–effectiveness analysis of trastuzumab for early breast cancer: a UK perspective considering duration of benefit, long-term toxicity and pattern of recurrence. Pharmacoeconomics 29:415–432. doi:10.2165/11588340-000000000-00000
Kondo M, Hoshi S-L, Yamanaka T et al (2011) Economic evaluation of the 21-gene signature (Oncotype DX) in lymph node-negative/positive, hormone receptor-positive early-stage breast cancer based on Japanese validation study (JBCRG-TR03). Breast Cancer Res Treat 127:739–749. doi:10.1007/s10549-010-1243-y
Whyte S, Cooper KL, Stevenson MD et al (2011) Cost–effectiveness of granulocyte colony-stimulating factor prophylaxis for febrile neutropenia in breast cancer in the United Kingdom. Value Health 14:465–474. doi:10.1016/j.jval.2010.10.037
Lux MP, Reichelt C, Karnon J et al (2011) Cost–benefit analysis of endocrine therapy in the adjuvant setting for postmenopausal patients with hormone receptor-positive breast cancer, based on survival data and future prices for generic drugs in the context of the German health care system. Breast Care (Basel) 6:381–389. doi:10.1159/000333118
Campbell HE, Epstein D, Bloomfield D et al (2011) The cost–effectiveness of adjuvant chemotherapy for early breast cancer: a comparison of no chemotherapy and first, second, and third generation regimens for patients with differing prognoses. Eur J Cancer 47:2517–2530. doi:10.1016/j.ejca.2011.06.019
Younis T, Rayson D, Skedgel C (2011) The cost–utility of adjuvant chemotherapy using docetaxel and cyclophosphamide compared with doxorubicin and cyclophosphamide in breast cancer. Curr Oncol 18:e288–e296
Athanasakis K, Kyriopoulos J (2012) A cost–effectiveness analysis of trastuzumab plus docetaxel vs. docetaxel alone for the treatment of HER2-positive metastatic breast cancer in the Greek healthcare setting. Forum Clin Oncol 3:28–34
Cheng TF, Wang JD, Uen WC (2012) Cost–utility analysis of adjuvant goserelin (Zoladex) and adjuvant chemotherapy in premenopausal women with breast cancer. BMC Cancer 12:33. doi:10.1186/1471-2407-12-33
Hannouf MB, Xie B, Brackstone M, Zaric GS (2012) Cost–effectiveness of a 21-gene recurrence score assay versus Canadian clinical practice in women with early-stage estrogen- or progesterone-receptor-positive, axillary lymph-node negative breast cancer. BMC Cancer 12:447. doi:10.1186/1471-2407-12-447
Hedden L, O’Reilly S, Lohrisch C et al (2012) Assessing the real-world cost–effectiveness of adjuvant trastuzumab in HER-2/neu positive breast cancer. Oncologist 17:164–171. doi:10.1634/theoncologist.2011-0379
Machado M, Einarson TR (2012) Lapatinib in patients with metastatic breast cancer following initial treatment with trastuzumab: an economic analysis from the Brazilian public health care perspective. Breast Cancer (Dove Med Press) 4:173–182. doi:10.2147/BCTT.S37003
Shih V, Chan A, Xie F, Ko Y (2012) Economic evaluation of anastrozole versus tamoxifen for early stage breast cancer in Singapore. Value Health Reg Issues 1:46–53. doi:10.1016/j.vhri.2012.03.013
Retèl VP, Joore MA, van Harten WH (2012) Head-to-head comparison of the 70-gene signature versus the 21-gene assay: cost–effectiveness and the effect of compliance. Breast Cancer Res Treat 131:627–636. doi:10.1007/s10549-011-1769-7
Hall PS, McCabe C, Stein RC, Cameron D (2012) Economic evaluation of genomic test-directed chemotherapy for early-stage lymph node-positive breast cancer. J Natl Cancer Inst 104:56–66. doi:10.1093/jnci/djr484
Bastani P, Kiadaliri AA (2012) Cost–utility analysis of adjuvant therapies for breast cancer in Iran. Int J Technol Assess Health Care 28:110–114. doi:10.1017/S0266462312000049
Montero AJ, Avancha K, Glück S, Lopes G (2012) A cost–benefit analysis of bevacizumab in combination with paclitaxel in the first-line treatment of patients with metastatic breast cancer. Breast Cancer Res Treat 132:747–751. doi:10.1007/s10549-011-1919-y
Ito K, Blinder VS, Elkin EB (2012) Cost effectiveness of fracture prevention in postmenopausal women who receive aromatase inhibitors for early breast cancer. J Clin Oncol 30:1468–1475. doi:10.1200/JCO.2011.38.7001
Kondo M, Hoshi S-L, Ishiguro H, Toi M (2012) Economic evaluation of the 70-gene prognosis-signature (MammaPrint®) in hormone receptor-positive, lymph node-negative, human epidermal growth factor receptor type 2-negative early stage breast cancer in Japan. Breast Cancer Res Treat 133:759–768. doi:10.1007/s10549-012-1979-7
Lamond NWD, Skedgel C, Rayson D et al (2012) Cost–utility of the 21-gene recurrence score assay in node-negative and node-positive breast cancer. Breast Cancer Res Treat 133:1115–1123. doi:10.1007/s10549-012-1989-5
Snedecor SJ, Carter JA, Kaura S, Botteman MF (2012) Cost–effectiveness of denosumab versus zoledronic acid in the management of skeletal metastases secondary to breast cancer. Clin Ther 34:1334–1349. doi:10.1016/j.clinthera.2012.04.008
Delea TE, Tappenden P, Sofrygin O et al (2012) Cost–effectiveness of lapatinib plus capecitabine in women with HER2+ metastatic breast cancer who have received prior therapy with trastuzumab. Eur J Health Econ 13:589–603. doi:10.1007/s10198-011-0323-1
Yang M, Rajan S, Issa AM (2012) Cost effectiveness of gene expression profiling for early stage breast cancer: a decision-analytic model. Cancer 118:5163–5170. doi:10.1002/cncr.27443
Blohmer JU, Rezai M, Kümmel S et al (2013) Using the 21-gene assay to guide adjuvant chemotherapy decision-making in early-stage breast cancer: a cost–effectiveness evaluation in the German setting. J Med Econ 16:30–40. doi:10.3111/13696998.2012.722572
Humphreys S, Pellissier J, Jones A (2013) Cost–effectiveness of an aprepitant regimen for prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer in the UK. Cancer Manag Res 5:215–224. doi:10.2147/CMAR.S44539
Lopes G, Glück S, Avancha K, Montero AJ (2013) A cost effectiveness study of eribulin versus standard single-agent cytotoxic chemotherapy for women with previously treated metastatic breast cancer. Breast Cancer Res Treat 137:187–193. doi:10.1007/s10549-012-2326-8
Das R, Cope S, Ouwens M et al (2013) Economic evaluation of fulvestrant 500 mg versus generic nonsteroidal aromatase inhibitors in patients with advanced breast cancer in the United Kingdom. Clin Ther 35(246–260):e5. doi:10.1016/j.clinthera.2013.01.011
Reed SD, Dinan MA, Schulman KA, Lyman GH (2013) Cost–effectiveness of the 21-gene recurrence score assay in the context of multifactorial decision making to guide chemotherapy for early-stage breast cancer. Genet Med 15:203–211. doi:10.1038/gim.2012.119
Alba E, Ciruelos E, López R et al (2013) Cost–utility analysis of nanoparticle albumin-bound paclitaxel versus paclitaxel in monotherapy in pretreated metastatic breast cancer in Spain. Expert Rev Pharmacoecon Outcomes Res 13:381–391. doi:10.1586/erp.13.18
Holt S, Bertelli G, Humphreys I et al (2013) A decision impact, decision conflict and economic assessment of routine Oncotype DX testing of 146 women with node-negative or pNImi, ER-positive breast cancer in the U.K. Br J Cancer 108:2250–2258. doi:10.1038/bjc.2013.207
Davidson JA, Cromwell I, Ellard SL et al (2013) A prospective clinical utility and pharmacoeconomic study of the impact of the 21-gene Recurrence Score® assay in oestrogen receptor positive node negative breast cancer. Eur J Cancer 49:2469–2475. doi:10.1016/j.ejca.2013.03.009
Ito K, Elkin E, Blinder V et al (2013) Cost–effectiveness of full coverage of aromatase inhibitors for Medicare beneficiaries with early breast cancer. Cancer 119:2494–2502. doi:10.1002/cncr.28084
Paulden M, Franek J, Pham B et al (2013) Cost–effectiveness of the 21-gene assay for guiding adjuvant chemotherapy decisions in early breast cancer. Value Health 16:729–739. doi:10.1016/j.jval.2013.03.1625
Buendía JA, Vallejos C, Pichón-Rivière A (2013) An economic evaluation of trastuzumab as adjuvant treatment of early HER2-positive breast cancer patients in Colombia. Biomedica 33:411–417
Garrison LP, Lalla D, Brammer M et al (2013) Assessing the potential cost–effectiveness of retesting IHC0, IHC1+, or FISH-negative early stage breast cancer patients for HER2 status. Cancer 119:3113–3122. doi:10.1002/cncr.28196
McCowan C, Wang S, Thompson AM et al (2013) The value of high adherence to tamoxifen in women with breast cancer: a community-based cohort study. Br J Cancer 109:1172–1180. doi:10.1038/bjc.2013.464
Delea TE, Amdahl J, Chit A, Amonkar MM (2013) Cost–effectiveness of lapatinib plus letrozole in her2-positive, hormone receptor-positive metastatic breast cancer in Canada. Curr Oncol 20:e371–e387. doi:10.3747/co.20.1394
Delea TE, Hawkes C, Amonkar MM et al (2013) Cost–effectiveness of lapatinib plus letrozole in post-menopausal women with hormone receptor- and HER2-positive metastatic breast cancer. Breast Care (Basel) 8:429–437. doi:10.1159/000357316
Retèl VP, Joore MA, Drukker CA et al (2013) Prospective cost–effectiveness analysis of genomic profiling in breast cancer. Eur J Cancer 49:3773–3779. doi:10.1016/j.ejca.2013.08.001
Yamauchi H, Nakagawa C, Yamashige S et al (2014) Societal cost–effectiveness analysis of the 21-gene assay in estrogen-receptor-positive, lymph-node-negative early-stage breast cancer in Japan. BMC Health Serv Res 14:372. doi:10.1186/1472-6963-14-372
Candon D, Healy J, Crown J (2014) Modelling the cost–effectiveness of adjuvant lapatinib for early-stage breast cancer. Acta Oncol 53:201–208. doi:10.3109/0284186X.2013.840740
Hannouf MB, Xie B, Brackstone M, Zaric GS (2014) Cost effectiveness of a 21-gene recurrence score assay versus Canadian clinical practice in post-menopausal women with early-stage estrogen or progesterone-receptor-positive, axillary lymph-node positive breast cancer. Pharmacoeconomics 32:135–147. doi:10.1007/s40273-013-0115-9
Erman A, Nugent A, Amir E, Coyte PC (2014) Cost–effectiveness analysis of extended adjuvant endocrine therapy in the treatment of post-menopausal women with hormone receptor positive breast cancer. Breast Cancer Res Treat 145:267–279. doi:10.1007/s10549-014-2950-6
Diaby V, Adunlin G, Zeichner SB et al (2014) Cost–effectiveness analysis of everolimus plus exemestane versus exemestane alone for treatment of hormone receptor positive metastatic breast cancer. Breast Cancer Res Treat 147:433–441. doi:10.1007/s10549-014-3042-3
Refaat T, Choi M, Gaber G et al (2014) Markov model and cost–effectiveness analysis of bevacizumab in HER2-negative metastatic breast cancer. Am J Clin Oncol 37:480–485. doi:10.1097/COC.0b013e31827e4e9a
Seguí MÁ, Crespo C, Cortés J et al (2014) Genomic profile of breast cancer: cost–effectiveness analysis from the Spanish National Healthcare System perspective. Expert Rev Pharmacoecon Outcomes Res 14:889–899. doi:10.1586/14737167.2014.957185
Aboutorabi A, Hadian M, Ghaderi H et al (2015) Cost–effectiveness analysis of trastuzumab in the adjuvant treatment for early breast cancer. Glob J Health Sci 7:98–106. doi:10.5539/gjhs.v7n1p98
Attard CL, Pepper AN, Brown ST et al (2015) Cost–effectiveness analysis of neoadjuvant pertuzumab and trastuzumab therapy for locally advanced, inflammatory, or early HER2-positive breast cancer in Canada. J Med Econ 18:173–188. doi:10.3111/13696998.2014.979938
National Institute for Health and Care Excellence (NICE). http://publications.nice.org.uk/pmg9. Accessed 24 March 2016
Guidelines for the economic evaluation of health technologies: Canada. https://www.cadth.ca/. Accessed 24 March 2016
Liberato NL, Marchetti M, Barosi G (2003) Clinical and economic issues in the treatment of advanced breast cancer with bisphosphonates. Drugs Aging 20:631–642
Benedict A, Brown RE (2005) Review of cost–effectiveness analyses in hormonal therapies in advanced breast cancer. Expert Opin Pharmacother 6:1789–1801. doi:10.1517/14656566.6.11.1789
Kilian R, Porzsolt F (2005) When to recommend and to pay for first-line adjuvant breast cancer treatment? A structured review of the literature. Breast 14:636–642. doi:10.1016/j.breast.2005.08.014
Dunn C, Keam SJ (2006) Letrozole: a pharmacoeconomic review of its use in postmenopausal women with breast cancer. Pharmacoeconomics 24:495–517
Karnon J (2006) Aromatase inhibitors in breast cancer: a review of cost considerations and cost effectiveness. Pharmacoeconomics 24:215–232
Norum J (2006) The cost–effectiveness issue of adjuvant trastuzumab in early breast cancer. Expert Opin Pharmacother 7:1617–1625. doi:10.1517/14656566.7.12.1617
Imai H, Kuroi K, Ohsumi S et al (2007) Economic evaluation of the prevention and treatment of breast cancer—present status and open issues. Breast Cancer 14:81–87
Younis T, Skedgel C (2008) Is trastuzumab a cost-effective treatment for breast cancer? Expert Rev Pharmacoecon Outcomes Res 8:433–442. doi:10.1586/14737167.8.5.433
Lwin Z, Leighl N (2009) Economic evaluation of docetaxel for breast cancer. Expert Opin Pharmacother 10:283–290. doi:10.1517/14656560802653206
Phillips KA, Marshall DA, Haas JS et al (2009) Clinical practice patterns and cost effectiveness of human epidermal growth receptor 2 testing strategies in breast cancer patients. Cancer 115:5166–5174. doi:10.1002/cncr.24574
Blank PR, Dedes KJ, Szucs TD (2010) Cost effectiveness of cytotoxic and targeted therapy for metastatic breast cancer: a critical and systematic review. Pharmacoeconomics 28:629–647. doi:10.2165/11535560-000000000-00000
Ferrusi IL, Leighl NB, Kulin NA, Marshall DA (2011) Do economic evaluations of targeted therapy provide support for decision makers? Am J Manag Care 17(Suppl 5 Developing):SP61–SP70
Lee JA, Shaheen M, Walke T, Daly M (2011) Clinical and health economic outcomes of alternative HER2 test strategies for guiding adjuvant trastuzumab therapy. Expert Rev Pharmacoecon Outcomes Res 11:325–341. doi:10.1586/erp.11.25
Glück S, Gorouhi F (2011) Clinical and economic benefits of aromatase inhibitor therapy in early-stage breast cancer. Am J Health Syst Pharm 68:1699–1706. doi:10.2146/ajhp100492
Foster TS, Miller JD, Boye ME et al (2011) The economic burden of metastatic breast cancer: a systematic review of literature from developed countries. Cancer Treat Rev 37:405–415. doi:10.1016/j.ctrv.2010.12.008
Frederix GWJ, Severens JL, Hövels AM et al (2012) Reviewing the cost–effectiveness of endocrine early breast cancer therapies: influence of differences in modeling methods on outcomes. Value Health 15:94–105. doi:10.1016/j.jval.2011.08.003
John-Baptiste AA, Wu W, Rochon P et al (2013) A systematic review and methodological evaluation of published cost–effectiveness analyses of aromatase inhibitors versus tamoxifen in early stage breast cancer. PLoS One 8:e62614. doi:10.1371/journal.pone.0062614
Rouzier R, Pronzato P, Chéreau E et al (2013) Multigene assays and molecular markers in breast cancer: systematic review of health economic analyses. Breast Cancer Res Treat 139:621–637. doi:10.1007/s10549-013-2559-1
Parkinson B, Pearson S-A, Viney R (2014) Economic evaluations of trastuzumab in HER2-positive metastatic breast cancer: a systematic review and critique. Eur J Health Econ 15:93–112. doi:10.1007/s10198-013-0459-2
Diaby V, Tawk R, Sanogo V et al (2015) A review of systematic reviews of the cost–effectiveness of hormone therapy, chemotherapy, and targeted therapy for breast cancer. Breast Cancer Res Treat 151:27–40. doi:10.1007/s10549-015-3383-6
Chan ALF, Leung HWC, Lu C-L, Lin SJ (2009) Cost–effectiveness of trastuzumab as adjuvant therapy for early breast cancer: a systematic review. Ann Pharmacother 43:296–303. doi:10.1345/aph.1L504
Shea BJ, Grimshaw JM, Wells GA et al (2007) Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 7:10. doi:10.1186/1471-2288-7-10
Shea BJ, Hamel C, Wells GA et al (2009) AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol 62:1013–1020. doi:10.1016/j.jclinepi.2008.10.009
Yang M, Patel DS, Tufail W, Issa AM (2013) The quality of economic studies of cancer pharmacogenomics: a quantitative appraisal of the evidence. Expert Rev Pharmacoecon Outcomes Res 13:597–611. doi:10.1586/14737167.2013.838023
Gonzalez-Perez JG (2002) Developing a scoring system to quality assess economic evaluations. Eur J Health Econ 3:131–136. doi:10.1007/s10198-002-0100-2
Gerkens S, Crott R, Cleemput I et al (2008) Comparison of three instruments assessing the quality of economic evaluations: a practical exercise on economic evaluations of the surgical treatment of obesity. Int J Technol Assess Health Care 24:318–325. doi:10.1017/S0266462308080422
Frederix GWJ, van Hasselt JGC, Schellens JHM et al (2014) The impact of structural uncertainty on cost–effectiveness models for adjuvant endocrine breast cancer treatments: the need for disease-specific model standardization and improved guidance. Pharmacoeconomics 32:47–61. doi:10.1007/s40273-013-0106-x
Briggs AH, Weinstein MC, Fenwick EAL et al (2012) Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-6. Value Health 15:835–842. doi:10.1016/j.jval.2012.04.014
Neumann PJ, Stone PW, Chapman RH et al (2000) The quality of reporting in published cost–utility analyses, 1976–1997. Ann Intern Med 132:964–972
Annemans L (2008) Methodological issues in evaluating cost effectiveness of adjuvant aromatase inhibitors in early breast cancer: a need for improved modelling to aid decision making. Pharmacoeconomics 26:409–423
Health economic evaluation publication guidelines (CHEERS): good reporting practices. http://www.ispor.org/Health-Economic-Evaluation-Publication-CHEERS-Guidelines.asp. Accessed 24 March 2016
Acknowledgments
No sources of funding were used to assist in the preparation of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest that are directly relevant to the content of this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nerich, V., Saing, S., Gamper, E.M. et al. Cost–utility analyses of drug therapies in breast cancer: a systematic review. Breast Cancer Res Treat 159, 407–424 (2016). https://doi.org/10.1007/s10549-016-3924-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3924-7