Abstract
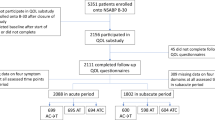
Adjuvant endocrine treatment-related adverse effects have a strong impact on patients’ quality of life and thereby limit therapy’s risk benefit ratio resulting in morbidity and treatment discontinuation. Still, many AI adverse effects remain untreated given that they are unrecognized by conservative methods (e.g., proxy ratings). The ability of complementary patient-reported outcomes (PROs) to provide a more comprehensive assessment of side-effects is to be explored. A cross-sectional study sample of 280 postmenopausal, early stage breast cancer patients was subjected to a comprehensive PRO assessment (FACT-B/+ES) at their after-care appointment. Prevalence and severity of patient-reported physical side-effects and psychosocial burden related to adjuvant AI therapy were compared with prevalences derived from pivotal phase IV trials (ATAC 2005, BIG1-98 2005). Across all symptom categories, highest prevalence rates were found for joint pain (59.6%), hot flushes (52%), lost interest in sexual intercourse (51.4%), and lack of energy (40.3%). Overall, PROs resulted in significantly higher prevalence rates as compared to physician ratings for all symptoms published in pivotal clinical trials except vaginal bleeding and nausea. The treatment duration exerted no significant impact on symptom frequency (P > 0.05). Established prevalence rates of endocrine treatment-related toxicity seem to be underestimated. The incorporation of PRO data should be mandatory or at least highly recommended in clinical treatment planning to arrive at a more accurate assessment of a patient’s actual symptom burden enabling improved individualized management of side-effects and mediating the preservation of treatment adherence.
Similar content being viewed by others
References
Early Breast Cancer Trialists’ Collaborative Group (1998) Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 351:1451–1467
Jonat W, Gnant M, Boccardo F et al (2006) Effectiveness of switching from adjuvant tamoxifen to anastrazole in postmenopausal women with hormone-sensitive early-stage breast cancer. Lancet Oncol 7:991–996
Howell A, Cuzick J, Baum M et al (2005) Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365(9453):60–62
The Breast International Group 1-98 Collaborative Group (2005) A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 353(26):2747–2757
Cuzick J, Sestak I, Baum M et al (2010) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol 11(12):1135–1141. doi:10.1016/S1470-2045(10)70257-6
World Health Organization (2002) Safety of Medicines. A guide to detecting and reporting adverse drug reactions. Why health professionals need to take action. http://whqlibdoc.who.int/hq/2002/WHO_EDM_QSM_2002.2.pdf. Accessed 19 Oct 2010
The ATAC Trialists’ Group (2002) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 359:2131–2139
Muss HB, Tu D, Ingle JN et al (2008) Efficacy, toxicity, and quality of life in older women with early-stage breast cancer treated with letrozole or placebo after 5 years of tamoxifen: NCIC CTG Intergroup Trial MA.17. J Clin Oncol 26(12):1956–1964
Coombes RC, Hall E, Gibson LJ et al (2004) A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 350(11):1081–1092
Fallowfield L (2007) Quality of life issues in relation to the aromatase inhibitor. J Steroid Biochem Mol Biol 106:168–172
Cella D, Fallowfield LJ (2008) Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat 107:167–180
Basch E (2010) The missing voice of patients in drug-safety reporting. N Engl J Med 362(10):865–869
Fellowes D, Fallowfield LJ, Saunders CM et al (2001) Tolerability of hormone therapies for breast cancer: how informative are documented symptom profiles in medical notes for ‘well-tolerated’ treatments? Breast Cancer Res Treat 66:73–81
Savage C, Pater JL, Tu D et al. (2002) He said/she said: how much agreement is there on symptoms between common toxicity criteria and quality of life? Proc Am Soc Clin Oncol 21(409):1540 (abstr)
Fallowfield LJ (2007) Why patient recorded outcomes should be mandatory in and outside clinical trials to guide management of patients with metastatic breast cancer. Breast Cancer Res 9(2):S7. doi:10.1186/bcr1805
Ruhstaller T, von Moos R, Rufibach K et al (2009) Breast cancer patients on endocrine therapy reveal more symptoms when self-reporting than in pivotal trials: an outcome research study. Oncology 76:142–148
Gibson L, Lawrence D, Dawson C et al. (2009) Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women. Cochrane Database Syst Rev 4 Art No: CD003370. doi: 10.1002/14651858.CD003370.pub3
US Department of Health and Human Services Food and Drug Administration Guidance for Industry (2009) Patient-reported outcome measures. Use in medical product development to support labeling claims. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf. Accessed 22 Oct 2010
Giesinger J, Kemmler G, Meraner V et al (2009) Towards the implementation of quality of life monitoring in daily clinical routine: methodological issues and clinical implication. Breast Care 4:148–154
ESD Inc (2010) E.S.D., Computer-based health evaluation system (CHES). Innsbruck, Austria
Brady MJ, Cella DF, Mo F et al (1997) Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. J Clin Oncol 15:974–986
Fallowfield LJ, Leaity SK, Howell A et al (1999) Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat 55:189–199
Cella D, Fallowfield L, Barker P et al (2006) Quality of life of postmenopausal women in the ATAC (“Arimidex”, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for early breast cancer. Breast Cancer Res Treat 100:273–284
Vollset SE (1993) Confidence intervals for a binomial proportion. Stat Med 12:809–827
Fallowfield LJ (2008) Treatment-decision making in breast cancer: the patient–doctor relationship. Breast Cancer Res Treat 112:5–13
Marquis P, Arnould B, Acquadro C, Roberts WM (2006) Patient-reported outcomes and health-related quality of life in effectiveness studies: pros and cons. Drug Develop Res 67:193–201
Cole SR, Stuart EA (2010) Generalizing evidence from randomized clinical trials to target populations: the ACTG 320 trial. Am J Epidemiol 172:107–115
Greenhouse JB, Kaizar EE, Kelleher K et al (2008) Generalizing from clinical trial data: a case study. The risk of suicidality among pediatric antidepressant users. Stat Med 27:1801–1813
Fraser J, Steele N, Zaman A, Yule A (2011) Are patients in clinical trials representative of the general population? Dose intensity and toxicities associated with FE100C-D chemotherapy in a non-trial population of node positive breast cancer patients compared with PACS-01 trial group. Eur J Cancer 47:215–220
Freedman OC, Verma S, Clemons MJ (2006) Pre-menopausal breast cancer and aromatase inhibitors: treating a new generation of women. Breast Cancer Res Treat 99:241–247
Fallowfield LJ, Cella D, Cuzick J et al (2004) Quality of life of postmenopausal women in the arimidex, tamoxifen, alone or in combination (ATAC) adjuvant breast cancer trial. J Clin Oncol 22(21):4261–4270
Jin J, Desta Z, Stearns V et al (2005) CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 97(1):30–39
Cleeland CS, Sloan JA (2010) Assessing the symptoms of cancer using patient-reported outcomes (ASCPRO): searching for standards. J Pain Symptom Manage 39(6):1077–1085
Doward LC, Gnanasakthy A, Baker MG (2010) Patient reported outcomes: looking beyond the label claim. Health Qual Life Outcomes 8:89
Partridge AH (2006) Non-adherence to endocrine therapy for breast cancer. Ann Oncol 17:183–184
Partridge AH, Avorn J, Wang PS et al (2002) Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst 94(9):652–661
Osterberg L, Blaschke T (2005) Adherence to medication. N Engl J Med 353:487–497
Snyder CF, Jensen RE, Geller G et al (2010) Relevant content for a patient-reported outcomes questionnaire for use in oncology clinical practice: putting doctors and patients on the same page. Qual Life Res 19:1045–1055
Acknowledgments
We would like to thank Prof. Dr. Wolfgang Fleischhacker from the University Clinic of Biological Psychiatry, Innsbruck Medical University for insightful comments. Monika Sztankay MSc and Anne Oberguggenberger MSc were financially supported by the Innsbruck Leopold-Franzens-University Innsbruck, Austria. This project was partly funded by AstraZeneca and Novartis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Anne Oberguggenberger and Michael Hubalek have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Oberguggenberger, A., Hubalek, M., Sztankay, M. et al. Is the toxicity of adjuvant aromatase inhibitor therapy underestimated? Complementary information from patient-reported outcomes (PROs). Breast Cancer Res Treat 128, 553–561 (2011). https://doi.org/10.1007/s10549-011-1378-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1378-5