Abstract
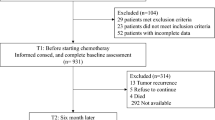
A Simplified Chinese version of the EORTC QLQ-BR53 was evaluated using responses from 233 patients with breast cancer in China by assessing the construct and criterion-related validity, internal consistency and test–retest reliability, and responsiveness as measured by score changes of the scales. Internal consistency reliability measured by Cronbach’s coefficient α is greater than 0.75 for most multi-item scales except cognitive functioning (0.41) and breast symptoms (0.71). Test–retest reliability coefficients for all domains are greater than 0.80 with the exception of physical functioning (0.65), social functioning (0.75), appetite loss (0.75), diarrhea (0.72), and body image (0.72). Correlation and factor analysis among domains and items showed good construct validity for both QLQ-C30 and QLQ-BR23. Score changes over time were observed in most domains except emotional functioning, global health status/QOL, dyspnoea, constipation, diarrhea, financial difficulties, sexual functioning, sexual enjoyment, and breast symptoms. Therefore, the Simplified Chinese version of QLQ-BR53 shows reasonable validity, reliability, and responsiveness and can be used to measure QOL for Chinese patients with breast cancer.
Similar content being viewed by others
References
Society AC (2000). Cancer facts and figures-2000. Atlanta
Coebergh JWW, Janssen-Heijnen MLG, Louwman WJ et al (2001) Cancer incidence and survival in the South of the Netherlands,1955–1999 and incidence in the North of Belgium, 1996–1998. Comprehensive Cancer Centre South (IKZ), Eindhoven
Selby PJ, Champon JA, Etazadi-Amoli J et al (1984) The development of a method for assessing the quality of life of cancer patients. Br J Cancer 50(1):13–22
Brade MJ, Cella DF, Mo F et al (1997) Reliability and validity of the functional assessment of cancer therapy-Breast quality-of- life instrument. J Clin Oncol 15(3):974–986
Cella DF, Tulsky DS, Gray G et al (1993) The functional assessment of cancer therapy scale development and validation of the general measure. J Clin Oncol 11(3):570–579
Aaronson NK, Cull A, Kaasa S et al (1994) The European Organization for Research and Treatment of Cancer (EORTC) modular approach to quality of life assessment in oncology. Int J Ment Health 23(2):75–96
Sprangers MA (1996) The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol 14(10):2756–2768
Van der Steeg AFW, De Vries J, Roukema JA (2004) Quality of life and health status in breast carcinoma. EJSO 30:1051–1057
Kim S, Hays RD, Birbeck GL et al (2003) Responsiveness of the Quality of Life in Epilepsy Inventory (QOLIE-89) in an Antiepileptic Drug Trial. Qual Life Res 12(2):147–155
Kosinski M, Bjorner JB, Ware Jr JE et al (2003) The Responsiveness of Headache Impact Scales Scored Using ‘Classical’ and ‘Modern’ Psychometric Methods: A Re-analysis of Three Clinical Trials. Qual Life Res 12(8):903–912
Yun YH, Bae SH, Kang IO et al (2004) Cross-cultural application of the Korean version of the European Organization for Research and Treatment of Cancer (EORTC) Breast-Cancer-Specific Quality of Life Questionnaire (EORTC QLQ-BR23). Support Care Cancer 12(6):441–445
Chie WC, Chang KJ, Huang CS, Kuo WH (2003) Quality of life of breast cancer patients in Taiwan: validation of the Taiwan Chinese version of the EORTC QLQ-C30 and EORTC QLQ-BR23. Psychooncology 12(7):729–735
Montazeri A, Harirchi I, Vahdani M et al (2000) The EORTC breast cancer-specific quality of life questionnaire (EORTC QLQ-BR23): translation and validation study of the Iranian version. Qual Life Res 9(2):177–184
Acknowledgements
This research is sponsored by the Natural Sciences Funds of Yunnan Province (99C0016G). In carrying out this research project, we not only received substantial help from the staff at the Oncological Hospital of Yunnan Province; but also received lots of help from Christiane van Pottelsberghethe, Karen West, at EORTC. We sincerely appreciate their kind help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wan, C., Tang, X., Tu, X.M. et al. Psychometric properties of the simplified Chinese version of the EORTC QLQ-BR53 for measuring quality of life for breast cancer patients . Breast Cancer Res Treat 105, 187–193 (2007). https://doi.org/10.1007/s10549-006-9443-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-006-9443-1