Abstract
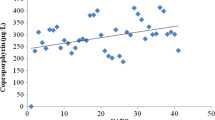
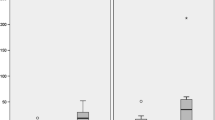
The study purpose was to compare the quantitative results from tests for urinary porphyrins, where some of these porphyrins are known biomarkers of heavy metal toxicity, to the independent assessments from a recognized quantitative measurement, the Autism Treatment Evaluation Checklist (ATEC), of specific domains of autistic disorders symptoms (Speech/Language, Sociability, Sensory/Cognitive Awareness, and Health/Physical/Behavior) in a group of children having a clinical diagnosis of autism spectrum disorder (ASD). After a Childhood Autism Rating Scale (CARS) evaluation to assess the development of each child in this study and aid in confirming their classification, and an ATEC was completed by a parent, a urinary porphyrin profile sample was collected and sent out for blinded analysis. Urinary porphyrins from twenty-four children, 2–13 years of age, diagnosed with autism or PDD-NOS were compared to their ATEC scores as well as their scores in the specific domains (Speech/Language, Sociability, Sensory/Cognitive Awareness, and Health/Physical/Behavior) assessed by ATEC. Their urinary porphyrin samples were evaluated at Laboratoire Philippe Auguste (which is an ISO-approved clinical laboratory). The results of the study indicated that the participants’ overall ATEC scores and their scores on each of the ATEC subscales (Speech/Language, Sociability, Sensory/Cognitive Awareness, and Health/Physical/Behavior) were linearly related to urinary porphyrins associated with mercury toxicity. The results show an association between the apparent level of mercury toxicity as measured by recognized urinary porphyrin biomarkers of mercury toxicity and the magnitude of the specific hallmark features of autism as assessed by ATEC.

Similar content being viewed by others
References
Adams JB, Romdalvik J, Ramanujam VM, Legator MS (2007) Mercury, lead, and zinc in baby teeth of children with autism versus controls. J Toxicol Environ Health A 70(12):1046–1051
Adams JB, Baral M, Geis E, Mitchel JL, Ingram J, Hensley A, Zappia I, Newmark S, Gehn E, Rubin R.A, Mitchell K, Bradstreet J, El-Dahr JJM (2009) The severity of autism is associated with toxic metal body burden and red blood cell glutathione levels. J Toxicol. doi:10.1155/2009/532640
American Psychiatric Association (2000) Diagnostic criteria for autistic disorder. In Diagnostic and statistical manual of mental disorders (Fourth edition—text revision (DSM-IV-TR). American Psychiatric Association, Washington, DC, p 75
Blaylock RL (2008) A possible central mechanism in autism spectrum disorders, part 1. Altern Ther in Health Med 14(6):46–53
Blaylock RL, Struneka A (2009) Immune-glutamatergic dysfunction as a central mechanism of the autism spectrum disorders. Curr Med Chem 16:157–170
Bradstreet J, Geier DA, Kartzinel JJ, Adams JB, Geier MR (2003) A case-control study of mercury burden in children with autistic spectrum disorders. J Am Phys Surg 8(3):76–79
Danscher G, Horsted-Bindslev P, Rungby J (1990) Traces of mercury in organs from primates with amalgam fillings. Exp Mol Pathol 52:291–299
DeSoto MC, Hitlan RT (2007) Blood levels of mercury are related to diagnosis of autism: a reanalysis of an important data set. J Child Neurol 22(11):1308–1311
Echeverria D, Heyer NJ, Martin MD, Naleway CA, Woods JS, Bittner AC (1995) Behavioral effects of low-level exposure to Hg0 among dentists. Neurotoxicol Teratol 17(2):161–168
Fonfria E, Vilaro MT, Babot Z, Rodriguez-Farre E, Sunol C (2005) Mercury compounds disrupt neuronal glutamate transport in cultured mouse cerebellar granule cells. J Neurosci Res 79(4):545–553
Hertz-Picciotto I (2009) Commentary: diagnostic change and the increase prevalence of autism. Int J Epidemiol 38(5):1239–1241
Hertz-Picciotto I, Delwiche L (2009) The rise in autism and the role of age at diagnosis. Epidemiology 20(1):84–90
Huang CF, Hsu CJ, Liu SH, Lin-Shiau SY (2008) Neurotoxicological mechanism of methylmercury induced by low-dose and long-term exposure in mice: oxidative stress and down-regulated Na+/K(+)-ATPase involved. Toxicol Lett 176(3):188–197
Kalia M (2008) Brain development: anatomy, connectivity, adaptive plasticity, and toxicity. Metabolism 57 Suppl 2:S2–S5
Kern JK (2003) Purkinje cell vulnerability and autism: a possible etiological connection. Brain Dev 25(6):377–382
Kern JK, Jones AM (2006) Evidence of toxicity, oxidative stress, and neuronal insult in autism. J Toxicol Environ Health B 9(6):485–499
Laks DR (2009) Assessment of chronic mercury exposure within the U.S. population, National Health and Nutrition Examination Survey, 1999–2006. Biometals 22(6):1103
Lopez-Hurtado E, Prieto JJ (2008) A microsopic study of language-related cortex in autism. Am J Biochem Biotechnol 4:130–145
Nataf R, Skorupka C, Amet L, Lam A, Springbett A, Lathe R (2006) Porphyinuria in childhood autistic disorder: implications for environmental toxicity. Toxicol Appl Pharmcol 214:99–108
Olanow CW, Arendash GW (1994) Metals and free radicals in neurodegeneration. Curr Opin Neurol 7:548–558
Pingree SD, Simmonds PL, Rummel KT, Woods JS (2001) Quantitative evaluation of urinary porphyrins as a measure of kidney mercury content and mercury body burden during prolonged methylmercury exposure in rats. Toxicol Sci 61(2):234–240
Rimland B, Edelson M (1999) Autism Treatment Evaluation Checklist. Autism Research Institute, 4812 Adams Ave, San Diego, CA 92116. www.ARI-ATEC.com and https://www.autismeval.com/ari-atec/report1.html
Rutter M (2005) Incidence of autism spectrum disorders: changes over time and their meaning. Acta Pediatr 94:2–15
Schopler E, Reichler RJ, Renner BR (1994) The Childhood Autism Rating Scale. Western Psychological Services, 12031 Wilshire Boulevard, Los Angeles, California, 90025-91251
Schuster PF, Krabbenhoft DP, Naftz DL, Cecil LD, Olson ML, Dewild JF, Susong DD, Green JR, Abbott ML (2002) Atmospherc mercury deposition during the last 270 years: a glacial ice core record of natural and anthropogenic sources. Environ Sci Technol 36(11):2303–2310
Stringari J, Nunes AK, Franco JL, Bohrer D, Garcia SC, Dafre AL, Milatovic D, Souza DO, Rocha JB, Aschner M, Farina M (2008) Prenatal methylmercury exposure hampers glutathione antioxidant system ontogenesis and causes long-lasting oxidative stress in the mouse brain. Toxicol Appl Pharmacol 227(1):147–154
Toimela T, Maenpaa H, Mannerstrom M, Tahti H (2004) Development of an in vitro blood-brain barrier model-cytotoxicity of mercury and aluminum. Toxicol Appl Pharmacol 195(1):73–82
Waly M, Olteanu H, Banjeree R, Choi SW, Manson JB, Parker BS, Sukumar S, Shim S, Sharma A, Benzecry JM, Power-Charnitsky VA, Deth RC (2004) Activation of methionine synthase by insulin-like growth factor-1 and dopamine: a target for neurodevelopmental toxins and thimerosal. Mol Psychiatry 9:358–370
Woods JS (1996) Altered prophyrin metabolism as a biomarker of mercury exposure and toxicity. Can J Physiol Pharmacol 74:210–215
Woods JS, Martin MD, Leroux BG, DeRouen TA, Bernardo MF, Luis HS, Leitao JG, Simmonds PL, Echeverria D, Rue TC (2009) Urinary porphyrins excretion in children with mercury amalgam treatment: findings from the Casa Pia Children’s Dental Amalgam Trial. J Toxicol Environ Health A 72(14):891–896
Acknowledgements
This research was funded by a grant from the Autism Research Institute, non-profit CoMeD, Inc., and by the non-profit Institute of Chronic Illnesses, Inc. through a grant from the Brenen Hornstein Autism Research & Education (BHARE) Foundation. The authors wish to acknowledge the help of the parents and children who participated in the study; without their participation this type of investigation would not be possible.
Conflict of interest statement
David Geier, Janet Kern, and Mark Geier have been involved in vaccine/biologic litigation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kern, J.K., Geier, D.A., Adams, J.B. et al. A biomarker of mercury body-burden correlated with diagnostic domain specific clinical symptoms of autism spectrum disorder. Biometals 23, 1043–1051 (2010). https://doi.org/10.1007/s10534-010-9349-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-010-9349-6