Abstract
Familial studies and reports of co-occurrence of gender identity disorder (GID) within a family may help to clarify the question of whether transsexualism is a familial phenomenon. In a sample of 995 consecutive transsexual probands (677 male-to-female [MF] and 318 female-to-male [FM]), we report 12 pairs of transsexual non-twin siblings (nine pairs of MF siblings, two pairs of MF-FM siblings, and one pair of FM siblings). The present study doubles the number of case reports of co-occurrence of transsexualism in non-twin siblings available in the literature. According to our data, the probability that a sibling of a transsexual will also be transsexual was 4.48 times higher for siblings of MF than for siblings of FM transsexual probands, and 3.88 times higher for the brothers than for the sisters of transsexual probands. Moreover, the prevalence of transsexualism in siblings of transsexuals (1/211 siblings) was much higher than the range expected according to the prevalence data of transsexualism in Spain. The study suggests that siblings of transsexuals may have a higher risk of being transsexual than the general population, and that the risk is higher for brothers than sisters of transsexuals, and for siblings of MF than FM transsexuals. Nevertheless, the risk is low.
Similar content being viewed by others
Introduction
Gender identity disorder (GID), as defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (American Psychiatric Association, 1994), has two main characteristics: a strong and persistent cross-gender identification and persistent discomfort with the individual’s assigned gender. The term GID in adolescents or adults, also referred to as transsexualism in the ICD-10 (World Health Organization, 1993), can be defined as an extreme form of gender dysphoria. Although the etiology of GID is unknown, it has been suggested that biological and environmental factors could contribute to gender identity variations (for a review, see Gooren, 2006). The biological line of research has focused on neuroanatomical (Kruijver et al., 2000; Zhou, Hofman, Gooren, & Swaab, 1995), hormonal (Swaab, 2004), and genetic influences. The research into genetic determinants has come from familial, twin, molecular, and chromosomal studies.
Familial studies and reports of co-occurrence of GID may help to partially explain the etiology of these disorders. Nevertheless, to our knowledge, no familial studies on transsexualism have been carried out and only several small series involving non-twin siblings have been published. There are reports of seven pairs of male-to-female (MF) (Ball, 1981; Green, 2000; Hore, Nicolle, & Calnan, 1973; Sabalis, Frances, Appenzeller, & Moseley, 1974; Stoller & Baker, 1973), two pairs of female-to-male (FM) (Green, 2000; Joyce & Ding, 1985), and one pair of MF-FM transsexual non-twin siblings (Green, 2000). The only published study of transsexual siblings or family members from a large sample (1,500 patients) was by Green (2000). Therefore, it is unknown whether there is a familial association on GID.
Twin studies are needed to disentangle the roles of genetic and environmental influences in the etiology of this disorder. Nevertheless, because the prevalence of transsexualism is low (Michel, Mormont, & Legros, 2001), these studies are scarce and are based mainly on twin case reports. In MF transsexualism, there are reports of six monozygotic (MZ) twin pairs concordant for transsexualism (Anchersen, 1956; Gooren, Frantz, Eriksson, & Rao, 1989; Green, 2000; Hyde & Kenna, 1977; Tsur, Borenstein, & Seidman, 1991; Zucker & Bradley, 1995), five MZ twin pairs discordant for transsexualism (Gooren et al., 1989; Green & Stoller, 1971; Hepp, Milos, & Braun-Sharm, 2004; Zucker & Bradley, 1995), and one report of two dizygotic (DZ) transsexual male triplets with a non-transsexual female co-triplet (McKee, Roback, & Hollender, 1976). In FM transsexual twins, one concordant (Sadeghi & Fakhrai, 2000) and four discordant MZ pairs (Garden & Rothery, 1992; Green & Stoller, 1971; Segal, 2006) have been reported. Moreover, most of the MZ and DZ twin case reports share not only genes but also many environmental factors. Therefore, it is not possible to identify the roles of different genetic influences on the basis of these case reports.
The heritability of GID or related traits (childhood gender nonconformity and atypical gender role development) has been assessed in three twin studies. In a retrospective study of 1,891 adult twins, Bailey, Dunne, and Martin (2000) found a significant heritable pattern for childhood gender nonconformity for both men and women, although the heritability estimates were stronger in men. In another study conducted with a sample of parents of 96 MZ and 61 DZ pairs of twins who completed a neuropsychological inventory to examine the prevalence of clinically significant GID symptomatology in their twins, Coolidge, Thede, and Young (2002) found that a genetic component accounted for 62% of the variance. Both studies support the hypothesis that childhood nonconformity and GID symptomatology have a strong heritable component. However, in a genetic study of atypical gender role development in 5,799 pairs of child-age twins, Knafo, Iervolino, and Plomin (2005) asked parents to rate the masculinity and femininity of their twins, and found that the extent of shared environmental effects was stronger than genetic effects in most cases (except for masculine girls, in whom group heritability accounted for most of the variance).
Molecular genetic studies can identify genetic markers of vulnerability or resilience. To our knowledge, three studies so far have reported the association between transsexualism and certain polymorphisms. All used case-control candidate gene association and focused on androgen and estrogen system genes. In a group of MF transsexuals who had been taking estrogens and anti-androgens for a minimum of 3 years, the distribution of the estrogenic receptor gene polymorphisms was similar to that in controls (Sosa et al., 2004). Henningsson et al. (2005) found significant partial effects for the risk of developing transsexualism on the interaction between three polymorphisms related to the androgen, beta estrogen, and aromatase genes. The data obtained in studies of transsexual populations indicate that the A2 variant of the CYP17 gene, which intervenes in the synthesis of dihydroepiandrosterone and 17-hydroprogesterone, might be involved in the etiology of MF transsexualism (Bentz et al., 2008). Recently, Hare et al. (2009) found a significant association between longer androgen receptor gene polymorphisms and MF transsexualism. However, these results should be considered with caution, since the statistical treatment was performed at the population level rather than the individual level.
Chromosomal abnormalities have also been found in transsexual individuals (for a review, see Swaab, 2004). Several cases of MF transsexuals with 47,XYY (Buhrich, Barr, & Lam-Po-Tang, 1978; Haberman, Hollingsworth, Falek, & Michael, 1975; Snaith, Penhale, & Horsfield, 1991; Taneja, Ammini, Mohapatra, Saxena, & Kucheria, 1992; Wagner, 1974) and female-to-male 47,XXX chromosome karyotypes have been reported (Turan et al., 2000). Because the 47,XYY karyotype occurs in approximately 1 in every 800 to 1000 male newborns, and the 47,XXX occurs in 1 in every 1000 female newborns (Grumbach, Hughes, & Conte, 2003), these cases may only represent a random association with transsexualism. Hengstschläger et al. (2003) analyzed G-banded karyotypes of 30 MF and 31 FM transsexuals and found no chromosomal aberration in these individuals with the exception of one balanced translocation 46,XY, and no evidence of molecular-cytogenetic alterations affecting either the androgen receptor gene region locus on chromosome Xq12 or the sex-determining region of the Y chromosome.
In summary, the evidence for genetic influences on GID from family, twin, molecular genetic, and chromosomal studies is limited. We concur with Green (2000) that the rarity of transsexualism makes studying families whose members show the phenomenon worthwhile, and that the creation of a database would contribute to research in this field.
The aim of our study was to describe the family co-occurrence of transsexualism in a sample of 995 Spanish transsexual patients in order to determine whether there was a familial association in non-twin siblings of individuals with transsexualism.
Method
Participants
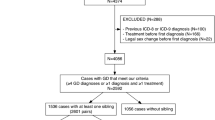
The study population comprised 995 transsexual probands (677 MF and 318 FM) from two gender identity disorders units in the Spanish public health system. One is in the region of Andalusia (Unidad de Trastornos de Identidad de Género (UTIG), Hospital Carlos Haya, Malaga) and the other in Catalonia (Unidad de Identidad de Género (UIG), Hospital Clínic, Barcelona) (Gómez-Gil & Esteva de Antonio, 2006). Both units provide specialized, comprehensive psychiatric-psychological and endocrine therapy for transsexual patients. Surgical treatment is provided from the UTIG since 2000, and from the UIG from January 2009. The database of each unit was set up in 2000 and this study included all patients evaluated as of March 2008. Both gender identity teams adopted the Standards of Care guidelines of the World Professional Association for Transgender Health (Meyer et al., 2001).
Measures and Procedure
Each patient completed several semi-structured clinical interviews performed independently by a psychiatrist and then a psychologist, both with several years of experience in GID diagnosis. The diagnoses were made using the DSM-IV (American Psychiatric Association, 1994) and ICD-10 criteria (World Health Organization, 1993). For all cases of GID included in this report, the two experts discussed the case prior to agreeing on the diagnosis.
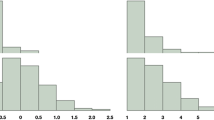
Sociodemographic, clinical, and psychiatric data that included any family background of GID were completed for all patients as part of similar standard clinical assessments at both clinics (Bergero Miguel et al., 2001; Esteva et al., 2001; Gómez-Gil, Trilla, Salamero, Godás, & Valdés, 2009). For patients with a family background of transsexualism, the variables selected from the clinical history were age at first request in the unit, age when they realized gender nonconformity (childhood onset), age at beginning hormonal therapy (with or without prescription), sex-reassignment surgeries, and sexual orientation. Sexual orientation was established by asking what partner (a man, a woman, both or neither) the patient would prefer or feel attraction to if they were completely free to choose and the body did not interfere. The same information about any relative who was reported as transsexual but had not been treated at the unit was obtained from the probands or family of probands.
Results
Male-to-Female Probands
Number of Transsexual Non-Twin Siblings
The main characteristics of transsexual sibling pairs are shown in Table 1. Of our 677 MF probands, we found 11 transsexual non-twin siblings (9 MF and 2 FM siblings).Footnote 1 The percentage of MF probands with a non-twin transsexual sibling was thus 1.6%.
Estimation of the Probability that a Sibling of a MF Transsexual will also be Transsexual
The number of biological siblings was obtained in a subsample of 333 probands. For this subsample, the total number of biological siblings through the mother was 883 (474 brothers and 409 sisters). Using an estimation of the number of biological siblings, for the total sample of 677 MF probands, the number of estimated biological siblings would be 1795 (n = 964 brothers and n = 831 sisters) (Table 2). Thus, the probability that a sibling of a MF transsexual would also be transsexual was 0.0061 (11:1795).
Female-to-Male Probands
Number of Transsexual Non-Twin Siblings
Of our 318 FM probands, we found one FM non-twin sibling (Table 1). Thus, the percentage of FM probands with a non-twin transsexual sibling was 0.3%, 5.3 times lower than the percentage of MF probands.
Estimation of the Probability that a Sibling of a FM Transsexual would also be Transsexual
For a subsample of 169 FM probands, we found 389 biological siblings (193 brothers and 196 sisters). Using an estimation of the number of biological siblings, for the total sample of 318 FM probands the number of biological siblings would be 732 (n = 363 brothers and n = 369 sisters). Thus, the probability that a sibling of a FM transsexual would also be transsexual was 0.0014 (1:732) (Table 2).
Total Probands
Differential Risk of Transsexualism in Brothers and Sisters of MF and FM Transsexuals
The probability that a sibling of a MF transsexual will also be transsexual (0.0061) was nearly 4.48 times higher than the probability for a sibling of a FM transsexual (0.0014). The probability that a sibling of a MF transsexual proband will also be transsexual was 3.88 times higher for the brothers (9:964) than for the sisters (2:831) (Table 2).Footnote 2
Prevalence of Transsexualism in Siblings of Transsexual Probands and Comparison with Prevalence Data in the General Population
The prevalence of transsexualism in siblings of transsexual probands was 1/211 (12:2527) (Table 2). According to health care demand, the prevalence of transsexualism in Andalusia has been estimated as 1/9,658 men for MF and 1/15,456 women for FM transsexualism (Esteva et al., 2006). In Catalonia, the prevalence has been estimated as 1/21,031 men and 1/48,096 women (Gómez-Gil et al., 2006). The estimated prevalence of transsexualism in siblings of transsexuals in our sample was much higher than that expected in the general population in Spain.Footnote 3
Discussion
The present case series adds nine cases of MF transsexual siblings to the seven already reported in the literature (Ball, 1981; Green, 2000; Hore et al., 1973; Sabalis et al., 1974; Stoller & Baker, 1973), two pairs of MF and FM transsexual siblings respectively to the case of MF transsexual with a gender dysphoric sister described by Green (2000), and a pair of FM transsexual siblings to the case also described by Green (2000). The present report doubles the existing literature of familial cases of non-twin transsexual siblings.
The only published study of sibling or family co-occurrence of gender dysphoria from a large sample of about 1,500 patients is by Green (2000). In his sample, Green found 6 pairs of transsexual siblings (5 pairs of non-twin and 1 pair of twin). In our study, from a sample of 995 patients, we found 14 pairs of transsexual siblings (12 non-twins and 2 twins). The higher proportion of cases in our study compared with Green’s study may be due to the fact that the total number of siblings in our population was larger than in his English population.
The estimated prevalence of transsexualism for non-twin siblings of transsexuals in our sample (1/211 siblings) is much higher than the prevalence of transsexualism expected in the general population according to the prevalence data of transsexualism in the Spanish population (Gómez-Gil et al., 2006). Spanish data on the prevalence of transsexualism were in line with previous literature (Michel et al., 2001). Therefore, our study data suggest that siblings of transsexuals are at a high risk for the disorder compared with the general population. Therefore, transsexualism may be familial. Nevertheless, the absolute percentage is quite low.
This result corroborates the fact that siblings of transsexuals are more likely than the general population to share factors that influence the disorder. Therefore, an etiological mechanism appears to be common in different populations. Nevertheless, family studies cannot tell us whether a disorder runs in families due to environmental factors, biological factors, or both. Twin studies are needed to identify the role of genetic and environmental influences in disorder etiology, but are limited to the case reports described above (Green, 2000; Segal, 2006), and to analyses of the heritability of gender nonconformity or sex-typed behaviors in children (Bailey et al., 2000; Coolidge et al., 2002; Knafo et al., 2005). Neither our data nor these studies identify the role of genetic or other influences in the etiology of the disorder.
Our ratio of MF:FM probands (2.13:1), in agreement with the literature (Michel et al., 2001), suggests a sex difference in the vulnerability to transsexualism. This sex difference is replicated in siblings of transsexuals. In fact, the probability that a sibling of a MF transsexual will also be transsexual was 3.88 times higher for brothers than sisters, and 4.48 times higher than in the case of a sibling of a FM transsexual. Male siblings and siblings of MF transsexuals are at a higher risk of transsexualism than female siblings and siblings of FM transsexuals. Green (2000) has suggested a genomic imprinting mechanism to explain the higher proportion of MF than FM transsexuals; if so, some genes in the chromosome may predispose the sons to feminization and the subsequent development of transsexualism. The possibility of a genetic biological factor in the etiology of at least some cases of transsexualism should be considered. Nevertheless, our results do not explain the etiology of transsexualism in a straightforward way. The sexual differentiation of the brain and behavior appears to be a very complex multi-signaling process involving genetic, hormonal, neural, and environmental variables (Segovia et al., 1999).
The present study had a number of limitations. First, the clinical assessment of family members with transsexualism or GID who were not patients at these clinics was collected indirectly from the transsexual probands, meaning that the diagnosis may be less reliable. Nevertheless, a diagnosis of transsexualism is highly probable since almost all these transsexual members were in hormonal therapy and had undergone vaginoplasty. Second, we did not analyze environmental influences. Nevertheless, studies reporting parental influences have no solid empirical support, and there are no studies of the influence of broader societal influences on GID (Cohen-Kettenis & Gooren, 1999). Moreover, the clinical impressions of the authors who attended transsexual patients are that these patients’ parents did not present more psychopathology, divorce, or familial trauma than the general population. Third, Blanchard and others have stressed the importance of distinguishing between homosexual and non-homosexual transsexuals, suggesting that each group has a possibly separate etiology (Blanchard & Sheridan, 1992). Since previous research in a Spanish population found high proportions of MF (89.9%) and FM (94.4%) transsexuals reporting same-sex sexual orientation (Gómez-Gil et al., 2009) compared with previous European studies (Lawrence, 2008), we did not consider that the lack of a separate analysis of sexual orientation would change our findings. Finally, the total number of biological siblings of the probands was estimated from the real number of siblings in a subsample of probands, which did not include those with a transsexual sibling.
In summary, we found a higher familial risk for transsexualism in siblings of transsexuals than in the general population, in siblings of MF than FM transsexuals, and in brothers than in sisters of transsexuals. As Green (2000) and Segal (2006) have noted, we also think that it is important to create a database of individuals with GID as well as a DNA bank of blood samples from these subjects (and mainly from familial cases with a large number of their family members), so as to be able to design future studies that might identify molecular markers. A bank of this kind would provide us with a sufficiently large number of cases to be able to propose verifiable hypotheses regarding the possible genetic cause of gender identity disorders in at least some families.
Notes
Data of other familial case reports from that population (two pairs of DZ twin siblings, eight pairs of first and second cousins, and three MF probands who have an uncle concordant for MF transsexualism) are available from the corresponding author upon request.
Data on siblings of FM probands were not compared, since only one familial case has been described.
Statistical comparison of two proportions was not performed because of the low prevalence data in the general population.
References
American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: Author.
Anchersen, P. (1956). Problems of transvestism. Acta Psychiatrica Neurologica Scandinavica, 106(Suppl), 249–256.
Bailey, J. M., Dunne, M. P., & Martin, N. G. (2000). Genetic and environmental influences on sexual orientation and its correlates in an Australian twin sample. Journal of Personality and Social Psychology, 78, 524–536.
Ball, J. R. (1981). Thirty years experience with transsexualism. Australia and New Zealand Journal of Psychiatry, 15, 39–43.
Bentz, E. K., Hefler, L. A., Kaufmann, U., Huber, J. C., Kolbus, A., & Tempfer, C. B. (2008). A polymorphism of the CYP17 gene related to sex steroid metabolism is associated with female-to-male transsexualism but not male-to-female transsexualism. Fertility and Sterility, 90, 56–59.
Bergero Miguel, T., Cano Oncala, G., Esteva de Antonio, I., Giraldo, F., Gornemann Schaffer, I., & Alvarez Ortega, P. (2001). Evaluación diagnóstica y seguimiento psicológico en la Unidad de Trastornos de Identidad de Género en Andalucía, Málaga [Psychological evaluation and follow-up by the Andalusian Gender Identity Disorder Unit in Malaga, Spain]. Cirugía Plástica Ibero-latinoamericana, 27, 263–272.
Blanchard, R., & Sheridan, P. (1992). Sibship size, sibling sex ratio, birth order, and parental age in homosexual and nonhomosexual gender dysphorics. Journal of Nervous and Mental Disease, 180, 40–47.
Buhrich, N., Barr, R., & Lam-Po-Tang, P. R. L. C. (1978). Two transsexuals with 47 XYY karyotype. British Journal of Psychiatry, 133, 77–81.
Cohen-Kettenis, P. T., & Gooren, L. J. G. (1999). Transsexualism: A review of etiology, diagnosis and treatment. Journal of Psychosomatic Research, 46, 315–333.
Coolidge, F. L., Thede, L. L., & Young, S. E. (2002). The heritability of gender identity disorder in a child and adolescent twin sample. Behavior Genetics, 32, 251–257.
Esteva, I., Giraldo, F., Bergero, T., Cano, G., Crespillo, C., Ruíz, S., et al. (2001). Evaluación endocrinológica y tratamiento hormonal de la transexualidad en la Unidad de Trastornos de Identidad de Género en Andalucía (Málaga) [Endocrinological evaluation and hormone treatment of transsexuality by the Andalusian Gender Identity Disorder Unit in Malaga (Spain)]. Cirugía Plástica Ibero-latinoamericana, 27, 273–280.
Esteva, I., Gonzalo, M., Yahyaoui, R., Domínguez, M., Berguero, T., & Giraldo, F. (2006). Epidemiología de la transexualidad en Andalucía: especial atención al grupo de adolescentes [Epidemiology of transsexualism in Andalusia: Special attention to the adolescent group]. Cuadernos de Medicina Psicosomática, 78, 65–70.
Garden, G. M., & Rothery, D. J. (1992). A female monozygotic twin pair discordant for transsexualism. Some theoretical implications. British Journal of Psychiatry, 161, 852–854.
Gómez-Gil, E., & Esteva de Antonio, I. (2006). Ser transexual [Being transsexual]. Barcelona: Editorial Glosa.
Gómez-Gil, E., Trilla, A., Salamero, M., Godás, T., & Valdés, M. (2009). Sociodemographic, clinical, and psychiatric characteristics of transsexuals from Spain. Archives of Sexual Behavior, 38, 378–392.
Gómez-Gil, E., Trilla-García, A., Godás-Sieso, T., Halperin-Rabinovich, I., Puig Domingo, M., Vidal Hagemeijer, A., et al. (2006). Estimación de la prevalencia, incidencia y razón de sexos del transexualismo en Cataluña según la demanda asistencial [Estimation of prevalence, incidence and sex ratio of transsexualism in Catalonia according to health care demand]. Actas Españolas de Psiquiatría, 34, 295–302.
Gooren, L. (2006). The biology of human psychosexual differentiation. Hormones and Behavior, 50, 589–601.
Gooren, L., Frantz, R. R., Eriksson, A. W., & Rao, B. R. (1989, August). Transsexualism in twins. Paper presented at the meeting of the International Congress of Twin Studies, Rome.
Green, R. (2000). Family cooccurrence of “gender dysphoria”: Ten sibling of parent-child pairs. Archives of Sexual Behavior, 29, 499–507.
Green, R., & Stoller, R. J. (1971). Two pairs of monozygotic (identical) twins discordant for gender identity. Archives of Sexual Behavior, 1, 321–328.
Grumbach, M. M., Hughes, I. A., & Conte, F. A. (2003). Disorders of sex differentiation. In P. R. Larsen, H. M. Kronenberg, S. Melmed, & K. S. Polonsky (Eds.), Williams textbook of endocrinology (10th ed., pp. 842–1002). Philadelphia: Saunders Company.
Haberman, M., Hollingsworth, F., Falek, A., & Michael, R. P. (1975). Gender identity confusion, schizophrenia and 47 XYY karyotype: A case report. Psychoneuroendocrinology, 1, 207–209.
Hare, L., Bernard, P., Sánchez, F. J., Baird, P. N., Vilain, E., Kennedy, T., et al. (2009). Androgen receptor repeat length polymorphism associated with male-to-female transsexualism. Biological Psychiatry, 65, 93–96.
Hengstschläger, M., van Trotsenburg, M., Repa, C., Marton, E., Huber, J. C., & Bernaschek, H. (2003). Sex chromosome aberrations and transsexualism. Fertility and Sterility, 79, 639–640.
Henningsson, S., Westberg, L., Nilsson, S., Lundström, B., Ekselius, L., Bodlund, O., et al. (2005). Sex steroid-related genes and male-to-female transsexualism. Psychoneuroendocrinology, 30, 657–664.
Hepp, U., Milos, G., & Braun-Sharm, H. (2004). Gender identity disorder and anorexia nervosa in male monozygotic twins. International Journal of Eating Disorders, 35, 239–243.
Hore, B. D., Nicolle, F. V., & Calnan, J. S. (1973). Male transsexualism: Two cases in a single family. Archives of Sexual Behavior, 2, 317–321.
Hyde, C., & Kenna, J. C. (1977). A male MZ twin pair, concordant for transsexualism, discordant for schizophrenia. Acta Psychiatrica Scandinavica, 56, 265–275.
Joyce, P., & Ding, L. (1985). Transsexual sisters. Australian and New Zealand Journal of Psychiatry, 19, 1988–1989.
Knafo, A., Iervolino, A. C., & Plomin, R. (2005). Masculine girls and feminine boys: Genetic and environmental contributions to atypical gender development in early childhood. Journal of Personality and Social Psychology, 88, 400–412.
Kruijver, F. P. M., Zhou, J. N., Pool, C. W., Hofman, M. A., Gooren, L. J. G., & Swaab, D. F. (2000). Male-to-female transsexuals have female neuron numbers in a limbic nucleus. Journal of Clinical Endocrinology and Metabolism, 85, 2034–2041.
Lawrence, A. A. (2008). Societal individualism predicts prevalence of nonhomosexual orientation in male-to-female transsexualism. Archives of Sexual Behavior. doi:10.1007/s10508-008-9420-3.
McKee, E. A., Roback, H. B., & Hollender, M. H. (1976). Transsexualism in two male triplets. American Journal of Psychiatry, 133, 334–340.
Meyer, W., Bockting, W., Cohen-Kettenis, P., Coleman, E., DiCeglie, D., Devor, H., et al. (2001). The Harry Benjamin Gender Dysphoria Association′s Standards of Care for Gender Identity Disorders, sixth version. Journal of Psychology and Human Sexuality, 13, 1–30.
Michel, A., Mormont, C., & Legros, J. J. (2001). A psycho-endocrinological overview of transsexualism. European Journal of Endocrinology, 145, 365–376.
Sabalis, R. F., Frances, A., Appenzeller, S. N., & Moseley, W. B. (1974). The three sisters: Transsexual male siblings. American Journal of Psychiatry, 131, 907–909.
Sadeghi, M., & Fakhrai, A. (2000). Transsexualism in female monozygotic twins: A case report. Australian and New Zealand Journal of Psychiatry, 34, 862–864.
Segal, N. (2006). Two monozygotic twin pairs discordant for female-to-male transsexualism. Archives of Sexual Behavior, 35, 347–358.
Segovia, S., Guillamon, A., del Cerro, M. C. R., Ortega, E., Perez-Laso, C., Rodríguez-Zafra, M., et al. (1999). The development of brain sex differences: A multisignaling process. Behavioral Brain Research, 105, 69–80.
Snaith, R. P., Penhale, S., & Horsfield, P. (1991). Male-to-female transsexual with XYY karyotype. Lancet, 337, 557–558.
Sosa, M., Jódar, E., Arbelo, E., Domínguez, C., Saavedra, P., Torres, A., et al. (2004). Serum lipids and estrogen receptor gene polymorphisms in male-to-female transsexuals: Effects of estrogen treatment. European Journal of Internal Medicine, 15, 231–237.
Stoller, R. J., & Baker, H. J. (1973). Two male transsexuals in one family. Archives of Sexual Behavior, 2, 323–328.
Swaab, D. F. (2004). Sexual differentiation of the human brain: Relevance for gender identity, transsexualism and sexual orientation. Gynecology and Endocrinology, 19, 301–312.
Taneja, N., Ammini, A. C., Mohapatra, I., Saxena, S., & Kucheria, K. (1992). A transsexual male with 47,XYY karyotype. British Journal of Psychiatry, 161, 698–699.
Tsur, H., Borenstein, A., & Seidman, D. S. (1991). Transsexualism. Lancet, 388, 945–946.
Turan, M. T., Esel, E., Dündar, M., Candemir, Z., Bastürk, M., Sofuoglu, S., et al. (2000). Female-to-male transsexual with 47,XXX karyotype. Biological Psychiatry, 48, 1116–1117.
Wagner, B. (1974). Ein transsexueller mit XYY syndrom. Nervenarzt, 45, 548–551.
World Health Organization. (1993). International classification of diseases: Diagnostic criteria for research (10th ed.). Geneva: Author.
Zhou, J. N., Hofman, M. A., Gooren, L. J. G., & Swaab, D. F. (1995). A sex difference in the human brain and its relation to transsexuality. Nature, 378, 68–70.
Zucker, K. J., & Bradley, S. J. (1995). Gender identity disorder and psychosexual problems in children and adolescents. New York: Guilford Press.
Acknowledgements
We are thankful to the patients and their families for their cooperation. The work of AG, EG, EP and SS is supported by grants SEJ2007-65686/PSIC, SAF2004-22551-E and PI-0254/2007 JA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gómez-Gil, E., Esteva, I., Almaraz, M.C. et al. Familiality of Gender Identity Disorder in Non-Twin Siblings. Arch Sex Behav 39, 546–552 (2010). https://doi.org/10.1007/s10508-009-9524-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10508-009-9524-4