Abstract
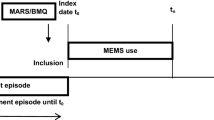
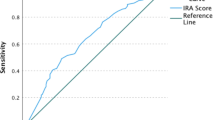
Few self-report measures of medication adherence have been rigorously developed and validated against electronic drug monitoring (EDM). Assess the validity of the 3-item self-report scale by comparing it with a contemporaneous EDM measure. We conducted an observational study in which adherence assessments were done monthly for up to 4 months for 81 patients with HIV who were taking antiretroviral medications. We report results for both HIV antiretroviral medications, and also for other, non-HIV-related medications. Raw and calibrated self-report adherence measures, electronic drug monitoring adherence measures, and sociodemographic variables. The mean age of patients was 46 years, 37 % were female, 49 % had some education beyond high school, 22 % were Black, and 22 % were Hispanic. Cronbach’s alphas for the 3-item scale for HIV and non-HIV medications were 0.83 and 0.87, respectively. The mean differences (raw/uncalibrated self-report scale minus EDM) for HIV and non-HIV medications were 7.5 and 5.2 points on a 100-point scale (p < 0.05 for both). Pearson correlation coefficients between the calibrated 3-item scale and the EDM for HIV and non-HIV medications were 0.47 and 0.59, respectively. The c-statistics for the ROC curves for the calibrated scale, using cut-offs of 0.8 and 0.9 for the EDM gold standard measure to define non-adherence, were between 0.74 and 0.76 for HIV and non-HIV medications. This 3-item adherence self-report scale showed good psychometric characteristics and good construct validity when compared with an EDM standard, for both HIV and non-HIV medications. In clinical care it can be a useful first-stage screener for non-adherence. In clinical research and quality improvement settings it can be a useful tool when more complex and expensive methods such as EDM or pharmacy claims are impractical or unavailable.



Similar content being viewed by others
References
Rand CS. “I took the medicine like you told me, Doctor”: self-reports of adherence with medical regimens. In: Stone AA, Turkkan JS, Bachrach CA, Jobe JB, Kurtzman HS, Cain VS, editors. The science of self-report: implications for research and practice, vol. 1. Mahwah: Lawrence Erlbaum Associates; 2000. p. 257–76.
Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–97.
Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105–16.
Demonceau J, Ruppar T, Kristanto P, et al. Identification and assessment of adherence-enhancing interventions in studies assessing medication adherence through electronically compiled drug dosing histories: a systematic literature review and meta-analysis. Drugs. 2013;73(6):545–62.
Kalichman SC, Amaral CM, Cherry C, et al. Monitoring medication adherence by unannounced pill counts conducted by telephone: reliability and criterion-related validity. HIV Clin Trials. 2008;9(5):298–308.
Wilson IB, Fowler FJ Jr, Cosenza CA, et al. Cognitive and field testing of a new set of medication adherence self-report items for HIV care. AIDS Behav. 2013;18(12):2349–58.
Fowler FJ, Lloyd SJ, Cosenza CA, Wilson IB. Coding cognitive interviews: an approach to enhancing the value of cognitive testing for survey question evaluation. Field Methods 2014;1525822X14549921.
Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74.
Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737–44.
Saunders JB, Aasland OG, Babor TF, De la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88(6):791–804.
Pence BW, Gaynes BN, Whetten K, Eron JJ Jr, Ryder RW, Miller WC. Validation of a brief screening instrument for substance abuse and mental illness in HIV-positive patients. J Acquir Immun Defic Syndr. 2005;40(4):434–44.
Genberg BL, Wilson IB, Bangsberg DR, et al. Patterns of antiretroviral therapy adherence and impact on HIV RNA among patients in North America. AIDS. 2012;26(11):1415–23.
Genberg BL, Lee Y, Rogers WH, Willey C, Wilson IB. Stages of change for adherence to antiretroviral medications. AIDS Patient Care STDS. 2013;27(10):567–72.
Genberg BL, Lee Y, Rogers WH, Wilson IB. Four types of barriers to adherence of antiretroviral therapy are associated with decreased adherence over time. AIDS Behav. 2015;19(1):85–92.
Gaito J. Measurement scales and statistics: resurgence of an old misconception. Psychol Bull. 1980;87(3):564–7.
Townsend JT, Hu GG, Evans RJ. Modeling feature perception in brief displays with evidence for positive interdependencies. Percept Psychophys. 1984;36(1):35–49.
Nunnally JC, Bernstein IH. Psychometric Theory. New York: McGraw-Hill, Inc.; 1994.
Thirumurthy H, Siripong N, Vreeman RC, et al. Differences between self-reported and electronically monitored adherence among patients receiving antiretroviral therapy in a resource-limited setting. AIDS. 2012;26(18):2399–403.
Shi LZ, Liu JN, Koleva Y, Fonseca V, Kalsekar A, Pawaskar M. Concordance of adherence measurement using self-reported adherence questionnaires and medication monitoring devices. Pharmacoeconomics. 2010;28(12):1097–107.
Shi L, Liu J, Fonseca V, Walker P, Kalsekar A, Pawaskar M. Correlation between adherence rates measured by MEMS and self-reported questionnaires: a meta-analysis. Health Qual Life Outcomes. 2010;8(1):99.
Amico KR, Marcus JL, McMahan V, et al. Study product adherence measurement in the iPrEx placebo-controlled trial: concordance with drug detection. J Acquir Immun Defic Syndr. 2014;66(5):530–7.
Wilson IB, Carter AE, Berg KM. Improving the self-report of HIV antiretroviral medication adherence: is the glass half full or half empty? CurrHIV/AIDS Rep. 2009;6(4):177–86.
Blair E, Burton S. Cognitive-processes used by survey respondents to answer behavioral frequency questions. J Consum Res. 1987;14(2):280–8.
Menon G. The effects of accessibility of information in memory on judgments of behavioral frequencies. J Consum Res. 1993;20(3):431–40.
Harrell FE, Lee KL, Mark DB. Tutorial in biostatistics multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87.
Wendel CS, Mohler MJ, Kroesen K, Ampel NM, Gifford AL, Coons SJ. Barriers to use of electronic adherence monitoring in an HIV clinic. Ann Pharmacother. 2001;35(9):1010–5.
Wilson IB, Tchetgen E, Spiegelman D. Patterns of adherence with antiretroviral medications: an examination of between-medication differences. J Acquir Immun Defic Syndr. 2001;28(3):259–63.
McNabb JJ, Nicolau DP, Stoner JA, Ross J. Patterns of adherence to antiretroviral medications: the value of electronic monitoring. AIDS. 2003;17(12):1763–7.
Gardner EM, Burman WJ, Maravi ME, Davidson AJ. Selective drug taking during combination antiretroviral therapy in an unselected clinic population. J Acquir Immun Defic Syndr. 2005;40(3):294–300.
Deschamps AE, De Geest S, Vandamme AM, Bobbaers H, Peetermans WE, van Wijngaerden E. Diagnostic value of different adherence measures using electronic monitoring and virologic failure as reference standards. AIDS Patient Care STDS. 2008;22(9):735–43.
Osterberg L, Urquhart J, Blaschke T. Understanding forgiveness: minding and mining the gaps between pharmacokinetics and therapeutics. Clin Pharmacol Ther. 2010;88(4):457–9.
Gulick RM. Adherence to antiretroviral therapy: how much is enough? Clin Infect Dis. 2006;43(7):943–4.
Kravitz RL, Melnikow J. Medical adherence research: time for a change in direction? Med Care. 2004;42(3):197–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the National Institute of Mental Health at the National Institutes of Health (Grant No. RO1 MH 092238). Dr. Wilson was also supported by a K24 Grant (2K24MH092242).
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
This work was presented in abstract form at the Pittsburgh Conference on the Science of Medication Adherence, Pittsburgh, PA. June 2, 2015.
Appendices
Appendix 1
In the last 30 days, on how many days did you miss at least one dose of any of your [drug name]?
Write in number of days: ____ (0–30)
In the last 30 days, how good a job did you do at taking your [drug name] in the way you were supposed to?
-
□ Very poor
-
□ Poor
-
□ Fair
-
□ Good
-
□ Very good
-
□ Excellent
In the last 30 days, how often did you take your [drug name] in the way you were supposed to?
-
□ Never
-
□ Rarely
-
□ Sometimes
-
□ Usually
-
□ Almost always
-
□ Always
Appendix 2
See Table 3.
Rights and permissions
About this article
Cite this article
Wilson, I.B., Lee, Y., Michaud, J. et al. Validation of a New Three-Item Self-Report Measure for Medication Adherence. AIDS Behav 20, 2700–2708 (2016). https://doi.org/10.1007/s10461-016-1406-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-016-1406-x