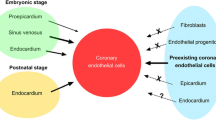
Abstract
The formation of the coronary vasculature involves a series of carefully regulated temporal events that include vasculogenesis, angiogenesis, arteriogenesis and remodeling. This review explores these events, which begin with the migration of proepicardial cells to form the epicardium and end with postnatal growth and remodeling. Coronary endothelial, smooth muscle and fibroblast cells differentiate via epithelial–mesenchymal transformation; these cells delaminate from the epicardium. Following the formation of a tubular network by endothelial cells, an aortic ring of endothelial cells penetrates the aorta at the left and right aortic cusps to form the two ostia. Smooth muscle cell recruitment occurs rapidly and the coronary artery network begins forming as blood flow is established. Recent studies have identified a number of regulatory molecules that play key roles in epicardial formation and the transformation of its component cells into mesenchyme. Moreover, we are finally gaining some understanding regarding the interplay of angiogenic growth factors in the complex process of establishing the coronary vascular tree. Understanding coronary embryogenesis is important for interventions regarding adult cardiovascular diseases as well as those necessary to correct congenital defects.
Similar content being viewed by others
References
Weinstein BM. What guides early embryonic blood vessel formation? Dev Dyn 1999; 215: 2–11
Tomanek RJ, Yue X, Zheng W. Vascular development of the heart. Boston: Birkhauser 2002
Bernanke DH, Velkey JM. Development of the coronary blood supply: Changing concepts and current ideas. Anat Rec 2002; 269: 198–208
Wada AM, Smith TK, Osler ME et al. Epicardial/mesothelial cell line retains vasculogenic potential of embryonic epicardium. Circ Res 2003; 92: 525–31
Wessels A, Perez-Pomares JM. The epicardium and epicardially derived cells (EPDCs) as cardiac stem cells. Anat Rec 2004; 276A: 43–57
Olivey HE, Compton LA, Barnett JV. Coronary vessel development: The epicardium delivers. Trends Cardiovasc Med 2004; 14: 247–51
Manasek FJ. Embryonic development of the heart. II. Formation of the epicardium. J Embryol Exp Morphol 1969; 22: 333–48
Manasek FJ. Histogenesis of the embryonic myocardium. Am J Cardiol 1970; 25: 149–68
Manner J, Perez-Pomares JM, Macias D, Munoz-Chapuli R. The origin, formation and developmental significance of the epicardium: A review. Cells Tissues Organs 2001; 169: 89–103
Viragh S, Challice CE. The origin of the epicardium and the embryonic myocardial circulation in the mouse. Anat Rec 1981; 201: 157–68
Watt AJ, Battle MA, Li J, Duncan SA. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc Natl Acad Sci USA 2004; 101: 12573–8
Viragh S, Challice CE. Origin and differentiation of cardiac muscle cells in the mouse. J Ultrastruct Res 1973; 42: 1–24
Komiyama M, Ito K, Shimada Y. Origin and development of the epicardium in the mouse embryo. Anat Embryol (Berl) 1987; 176: 183–9
Ho E, Shimada Y. Formation of the epicardium studied with the scanning electron microscope. Dev Biol 1978; 66: 579–85
Hiruma T, Hirakow R. Epicardial formation in embryonic chick heart: Computer-aided reconstruction, scanning, and transmission electron microscopic studies. Am J Anat 1989; 184: 129–38
Manner J. The development of pericardial villi in the chick embryo. Anat Embryol (Berl) 1992; 186: 379–85
Manner J. Experimental study on the formation of the epicardium in chick embryos. Anat Embryol (Berl) 1993; 187: 281–9
Viragh S, Gittenberger-de Groot AC, Poelmann RE, Kalman F. Early development of quail heart epicardium and associated vascular and glandular structures. Anat Embryol (Berl) 1993; 188: 381–93
Vrancken Peeters M-PFM, Mentink MMT, Poelmann RE, Gittenberger-de Groot AC. Cytokeratins as a marker for epicardial formation in the quail embryo. Anat Embryol (Berl) 1995; 191: 503–8
Van den Eijnde SM, Wenink AC, Vermeij-Keers C. Origin of subepicardial cells in rat embryos. Anat Rec 1995; 242: 96–102
Munoz-Chapuli R, Macias D, Ramos C et al. Cardiac development in the dogfish (Scyliorhinus canicula): A model for the study of vertebrate cardiogenesis. Cardioscience 1994; 5: 245–53
Pinco KA, Liu S, Yang JT. alpha4 integrin is expressed in a subset of cranial neural crest cells and in epicardial progenitor cells during early mouse development. Mech Dev 2001; 100: 99–103
Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by α4 integrins are essential in placental and cardiac development. Development 1995; 121: 549–60
Kwee L, Baldwin HS, Shen HM et al. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development 1995; 121: 489–503
Kalman F, Viragh S, Modis L. Cell surface glycoconjugates and the extracellular matrix of the developing mouse embryo epicardium. Anat Embryol (Berl) 1995; 191: 451–64
Hidai H, Bardales R, Goodwin R et al. Cloning of capsulin, a basic helix–loop–helix factor expressed in progenitor cells of the pericardium and the coronary arteries. Mech Dev 1998; 73: 33–43
Robb L, Mifsud L, Hartley L et al. Epicardin: A novel basic helix–loop–helix transcription factor gene expressed in epicardium, branchial arch myoblasts, and mesenchyme of developing lung, gut, kidney, and gonads. Dev Dyn 1998; 213: 105–13
Li WE, Waldo K, Linask KL et al. An essential role for connexin43 gap junctions in mouse coronary artery development. Development 2002; 129: 2031–42
Walker DL, Vacha SJ, Kirby ML, Lo CW. An essential role for connexin 43 in coronary vasculogenesis. Mol Biol Cell 2004 15(Suppl1): 312a
Wu H, Lee SH, Gao J et al. Inactivation of erythropoietin leads to defects in cardiac morphogenesis. Development 1999; 126: 3597–605
Wada AM, Reese DE, Bader DM. Bves: Prototype of a new class of cell adhesion molecules expressed during coronary artery development. Development 2001; 128: 2085–93
Perez-Pomares JM, Macias D, Garcia-Garrido L, Munoz-Chapuli R. Contribution of the primitive epicardium to the subepicardial mesenchyme in hamster and chick embryos. Dev Dyn 1997; 210: 96–105
Dettman RW, Denetclaw W, Jr, Ordahl CP, Bristow J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev Biol 1998; 193: 169–81
Bouchey D, Drake CJ, Wunsch AM, Little CD. Distribution of connective tissue proteins during development and neovascularization of the epicardium. Cardiovasc Res 1996; 31 Spec No: E104–115
Burch GH, Bedolli MA, McDonough S et al. Embryonic expression of tenascin-X suggests a role in limb, muscle, and heart development. Dev Dyn 1995; 203: 491–504
Viragh SZ, Kalman F, Gittenberger-de Groot AC et al. Angiogenesis and hematopoiesis in the epicardium of the vertebrate embryo heart. Ann NY Acad Sci 1990; 588: 455–458
Munoz-Chapuli R, Perez-Pomares JM, Macias D et al. Differentiation of hemangioblasts from embryonic mesothelial cells? A model on the origin of the vertebrate cardiovascular system. Differentiation 1999; 64: 133–41
Kattan J, Dettman RW, Bristow J. Formation and remodeling of the coronary vascular bed in the embryonic avian heart. Dev Dyn 2004; 230: 34–43
Moore AW, McInnes L, Kreidberg J et al. YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development 1999; 126: 1845–57
Kreidberg JA, Sariola H, Loring JM et al. WT-1 is required for early kidney development. Cell 1993; 74: 679–91
Eralp I, Lie-Venema H, DeRuiter MC et al. Coronary artery and orifice development is associated with proper timing of epicardial outgrowth and correlated fas ligand associated apoptosis patterns. Circ Res 2005; 96: 526–34
Morabito CJ, Dettman RW, Kattan J et al. Positive and negative regulation of epicardial–mesenchymal transformation during avian heart development. Dev Biol 2001; 234: 204–15
Ward NL, Van Slyke P, Sturk C et al. Angiopoietin 1 expression levels in the myocardium direct coronary vessel development. Dev Dyn 2004; 229: 500–9
Tevosian SG, Deconinck AE, Tanaka M et al. FOG-2, a cofactor for GATA transcription factors, is essential for heart morphogenesis and development of coronary vessels from epicardium. Cell 2000; 101: 729–39
Lie-Venema H, Gittenberger-de Groot AC, van Empel LJ et al. Ets-1 and Ets-2 transcription factors are essential for normal coronary and myocardial development in chicken embryos. Circ Res 2003; 92: 749–56
Heintzberger CF. Development of myocardial vascularisation in the rat. Acta Morphol Neerl Scand 1983; 21: 267–84
Mikawa T, Fischman DA. Retroviral analysis of cardiac morphogenesis: Discontinuous formation of coronary vessels. Proc Natl Acad Sci USA 1992; 89: 9504–8
Poelmann RE, Gittenberger-de Groot AC, Mentink MM et al. Development of the cardiac coronary vascular endothelium, studied with antiendothelial antibodies, in chicken-quail chimeras. Circ Res 1993; 73: 559–68
Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol 1996; 174: 221–32
Perez-Pomares JM, Macias D, Garcia-Garrido L, Munoz-Chapuli R. The origin of the subepicardial mesenchyme in the avian embryo: An immunohistochemical and quail-chick chimera study. Dev Biol 1998; 200: 57–68
Vrancken Peeters M-PFM, Gittenberger-de Groot AC, Mentink MMT PR. Smooth muscle cells and fibroblasts of the coronary arteries derive from epithelial–mesenchymal transformation of the epicardium. Anat Embryol (Berl) 1999; 199: 367–78
Nahirney PC, Mikawa T, Fischman DA. Evidence for an extracellular matrix bridge guiding proepicardial cell migration to the myocardium of chick embryos. Dev Dyn 2003; 227: 511–23
Young PP, Hofling AA, Sands MS. VEGF increases engraftment of bone marrow-derived endothelial progenitor cells (EPCs into vasculature of newborn murine recipients. Proc Natl Acad Sci USA 2002; 99: 11951–6
Rongish BJ, Torry RJ, Tucker DC, RJ T. Neovascularization of embryonic rat hearts cultured in oculo closely mimics in utero coronary vessel development. J Vas Res 1994; 31: 206–15
Perez-Pomares JM, Macias D, Garcia-Garrido L, Munoz-Chapuli R. Immunolocalization of the vascular endothelial growth factor receptor-2 in the subepicardial mesenchyme of hamster embryos: Identification of the coronary vessel precursors. Histochem J 1998; 30: 627–34
Perez-Pomares JM, Carmona R, Gonzalez-Iriarte M et al. Origin of coronary endothelial cells from epicardial mesothelium in avian embryos. Int J Dev Biol 2002; 46: 1005–13
Manner J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. Anat Rec 1999; 255: 212–26
Partanen TA, Makinen T, Arola J et al. Endothelial growth factor receptors in human fetal heart. Circulation 1999; 100: 583–6
Tsuda T, Wang H, Timpl R, Chu ML. Fibulin-2 expression marks transformed mesenchymal cells in developing cardiac valves, aortic arch vessels, and coronary vessels. Dev Dyn 2001; 222: 89–100
Tomanek RJ, Ratajska A, Kitten GT et al. Vascular endothelial growth factor expression coincides with coronary vasculogenesis and angiogenesis. Dev Dyn 1999; 215: 54–61
Rongish BJ, Hinchman G, Doty MK et al. Relationship of the extracellular matrix to coronary neovascularization during development. J Mol Cell Cardiol 1996; 28: 2203–15
Hirakow R. Development of the cardiac blood vessels in staged human embryos. Acta Anat (Basel) 1983; 115: 220–30
Tomanek RJ, Hu N, Phan B, Clark EB. Rate of coronary vascularization during embryonic chicken development is influenced by the rate of myocardial growth. Cardiovasc Res 1999; 41: 663–71
Ratajska A, Ciszek B, Sowinska A. Embryonic development of coronary vasculature in rats: corrosion casting studies. Anat Rec 2003; 270A: 109–16
Rychter Z, Ostadal B. Mechanism of the development of coronary arteries in chick embryo. Folia Morphol (Praha) 1971; 19: 113–24
Dbaly J, Ostadal B, Rychter Z. Development of the coronary arteries in rat embryos. Acta Anat 1968; 71: 209–22
Voboril Z, Schiebler TH. Development of blood supply in the rat heart. Z Anat Entwicklungsgesch 1969; 129: 24–40
Tomanek RJ, Haung L, Suvarna PR et al. Coronary vascularization during development in the rat and its relationship to basic fibroblast growth factor. Cardiovasc Res 1996; 31 Spec No: E116–26
Porter GA, Bankston PW. Maturation of myocardial capillaries in the fetal and neonatal rat: an ultrastructural study with a morphometric analysis of the vesicle populations. Am J Anat 1987; 178: 116–25
Oštádal B, Schiebler T, Rychter Z. Relations between the development of the capillary wall and myoarchitecture of the rat heart. Adv Exp Med Biol 1975; 53: 375–88
Tomanek RJ, Lotun K, Clark EB et al. VEGF and bFGF stimulate myocardial vascularization in embryonic chick. Am J Physiol 1998; 274: H1620–1626
Joseph-Silverstein J, Consigli SA, Lyser KM, Ver Pault C. Basic fibroblast growth factor in the chick embryo: Immunolocalization to striated muscle cells and their precursors. J Cell Biol 1989; 108: 2459–66
Spirito P, Fu YM, Yu ZX et al. Immunohistochemical localization of basic and acidic fibroblast growth factors in the developing rat heart. Circulation 1991; 84: 322–32
Ratajska A, Torry RJ, Kitten GT et al. Modulation of cell migration and vessel formation by vascular endothelial growth factor and basic fibroblast growth factor in cultured embryonic heart. Dev Dyn 1995; 203: 399–407
Bolender D, Olson M, Markwald R. Coronary vessel vasculogenesis. Ann NY Acad Sci 1990; 588: 340–4
Yue X, Tomanek RJ. Stimulation of coronary vasculogenesis/angiogenesis by hypoxia in cultured embryonic hearts. Dev Dyn 1999; 216: 28–36
Yue X, Tomanek RJ. Effects of VEGF(165) and VEGF(121) on vasculogenesis and angiogenesis in cultured embryonic quail hearts. Am J Physiol Heart Circ Physiol 2001; 280: H2240–2247
Favier J, Kempf H, Corvol P, Gasc JM. Coexpression of endothelial PAS protein 1 with essential angiogenic factors suggests its involvement in human vascular development. Dev Dyn 2001; 222: 377–88
Mancini L, Bertossi M, Ribatti D et al. The effects of long-term hypoxia on epicardium and myocardium in developing chick embryo hearts. Int J Microcirc Clin Exp 1991; 10: 359–71
Tomanek RJ, Zheng W, Peters KG et al. Multiple growth factors regulate coronary embryonic vasculogenesis. Dev Dyn 2001; 221: 265–73
Lagercrantz J, Farnebo F, Larsson C et al. A comparative study of the expression patterns for vegf, vegf-b/vrf and vegf-c in the developing and adult mouse. Biochim Biophys Acta 1998; 1398: 157–63
Ikuta T, Ariga H, Matsumoto KI. Effect of tenascin-X together with vascular endothelial growth factor A on cell proliferation in cultured embryonic hearts. Biol Pharm Bull 2001; 24: 1320–3
Achen MG, Jeltsch M, Kukk E et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci USA 1998; 95: 548–53
Makinen T, Olofsson B, Karpanen T et al. Differential binding of vascular endothelial growth factor B splice and proteolytic isoforms to neuropilin-1. J Biol Chem 1999; 274: 21217–22
Lymboussaki A, Olofsson B, Eriksson U, Alitalo K. Vascular endothelial growth factor (VEGF) and VEGF-C show overlapping binding sites in embryonic endothelia and distinct sites in differentiated adult endothelia. Circ Res 1999; 85: 992–9
Pepper M, Mandriota S, Jeltsch M et al. Vascular endothelial growth factor (VEGF)-C synergizes with basic fibroblast growth factor and VEGF in the induction of angiogenesis in vitro and alters endothelial cell extracellular proteolytic activity. J Cell Physiol 1998; 177: 439–52
Tomanek RJ, Holifield JS, Reiter RS et al. Role of VEGF family members and receptors in coronary vessel formation. Dev Dyn 2002; 225: 233–40
Vokes SA, Yatskievych TA, Heimark RL et al. Hedgehog signaling is essential for endothelial tube formation during vasculogenesis. Development 2004; 131: 4371–80
Holifield JS, Arlen AM, Runyan RB, Tomanek RJ. TGF-beta(1), -beta(2) and -beta(3) cooperate to facilitate tubulogenesis in the explanted quail heart. J Vasc Res 2004; 41: 491–8
Landerholm TE, Dong XR, Lu J et al. A role for serum response factor in coronary smooth muscle differentiation from proepicardial cells. Development 1999; 126: 2053–62
Majesky MW. Decisions, decisions...SRF coactivators and smooth muscle myogenesis. Circ Res 2003; 92: 824–6
Yoshida T, Sinha S, Dandre F et al. Myocardin is a key regulator of CArG-dependent transcription of multiple smooth muscle marker genes. Circ Res 2003; 92: 856–64
Wang D, Chang PS, Wang Z et al. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell 2001; 105: 851–62
Chang DF, Belaguli NS, Iyer D et al. Cysteine-rich LIM-only proteins CRP1 and CRP2 are potent smooth muscle differentiation cofactors. Dev Cell 2003; 4: 107–18
Nelson TJ, Duncan SA, Misra RP. Conserved enhancer in the serum response factor promoter controls expression during early coronary vasculogenesis. Circ Res 2004; 94: 1059–66
Lu J, Landerholm TE, Wei JS et al. Coronary smooth muscle differentiation from proepicardial cells requires rhoA-mediated actin reorganization and p160 rho-kinase activity. Dev Biol 2001; 240: 404–18
Reese DE, Zavaljevski M, Streiff NL, Bader D. bves: A novel gene expressed during coronary blood vessel development. Dev Biol 1999; 209: 159–71
Munoz-Chapuli R, Macias D, Ramos C et al. Development of the subepicardial mesenchyme and the early cardiac vessels in the dogfish (Scyliorhinus canicula). J Exp Zool 1996; 275: 95–111
Vrancken Peeters M-PFM, Gittenberger-de Groot AC, Mentink MMT et al. The development of the coronary vessels and their differentiation into arteries and veins in the embryonic quail heart. Dev Dyn 1997; 208: 338–48
Bennet HS. The development of the blood supply to the heart in the embryo pig. Am J Anat 1936; 60: 27–53
Goldsmith JB, HW B. The development of the cardiac–coronary circulatory system. Am J Anat 1937; 60: 185–201
Grant R. Development of the cardiac coronary vessels in the rabbit. Heart 1926; 13: 261–71
Bogers AJ, Gittenberger-de Groot AC, Poelmann RE et al. Development of the origin of the coronary arteries, a matter of ingrowth or outgrowth? Anat Embryol (Berl) 1989; 180: 437–41
Waldo KL, Willner W, Kirby ML. Origin of the proximal coronary artery stems and a review of ventricular vascularization in the chick embryo. Am J Anat 1990; 188: 109–20
Ando K, Nakajima Y, Yamagishi T et al. Development of proximal coronary arteries in quail embryonic heart: Multiple capillaries penetrating the aortic sinus fuse to form main coronary trunk. Circ Res 2004; 94: 346–52
Velkey JM, Bernanke DH. Apoptosis during coronary artery orifice development in the chick embryo. Anat Rec 2001; 262: 310–7
Ratajska A, Fiejka E. Prenatal development of coronary arteries in the rat: Morphologic patterns. Anat Embryol (Berl) 1999; 200: 533–40
Ratajska A, Fiejka E, Sieminska J. Prenatal development of coronary arteries in the rat: morphometric patterns. Folia Morphol (Warsz) 2000; 59: 297–306
Harris BS, O’Brien TX, Gourdie RG. Coronary arteriogenesis and differentiation of periarterial Purkinje fibers in the chick heart: Is there a link? Tex Heart Inst J 2002; 29: 262–70
Ratajska A, Zarska M, Quensel C, Kramer J. Differentiation of the smooth muscle cell phenotypes during embryonic development of coronary vessels in the rat. Histochem Cell Biol 2001; 116: 79–87
Hood LC, Rosenquist TH. Coronary artery development in the chick: Origin and deployment of smooth muscle cells, and the effects of neural crest ablation. Anat Rec 1992; 234: 291–300
Boucek RJ MA, Romanelli R, Judkins MP. Embryology and congenital anomalies of the coronary arteries. Coronary Artery Disease. Pathological and Clinical Assessment: Baltimore/London 1984
Hutchins GM, Kessler-Hanna A, Moore GW. Development of the coronary arteries in the embryonic human heart. Circulation 1988; 77: 1250–7
Mandarim-de-Lacerda PC. Growth of the heart in Brazilian fetuses. Anat Anz 1990; 170: 15–9
Waldo KL, Kumiski DH, Kirby ML. Association of the cardiac neural crest with development of the coronary arteries in the chick embryo. Anat Rec 1994; 239: 315–31
Jiang X, Rowitch DH, Soriano P et al. Fate of the mammalian cardiac neural crest. Development 2000; 127: 1607–16
Torry RJ, Rongish BJ, Tucker DC et al. Influence of graft innervation on neovascularization of embryonic heart tissue grafted in oculo. Am J Physiol 1996; 270: H33–37
Gonzalez-Iriarte M, Carmona R, Perez-Pomares JM et al. Development of the coronary arteries in a murine model of transposition of great arteries. J Mol Cell Cardiol 2003; 35: 795–802
Olivetti G, Anversa P, Loud AV. Morphometric study of early postnatal development in the left and right ventricular myocardium of the rat. II. Tissue composition, capillary growth, and sarcoplasmic alterations. Circ Res 1980; 46: 503–12
Smolich JJ, Walker AM, Campbell GR, Adamson TM. Left and right ventricular myocardial morphometry in fetal, neonatal, and adult sheep. Am J Physiol 1989; 257: H1–9
Rakusan K, Turek Z. Protamine inhibits capillary formation in growing rat hearts. Circ Res 1985; 57: 393–8
Anversa P, Capasso JM, Ricci R et al. Morphometric analysis of coronary capillaries during physiologic myocardial growth and induced cardiac hypertrophy: a review. Int J Microcirc Clin Exp 1989; 8: 353–63
Tomanek RJ, Sandra A, Zheng W et al. Vascular endothelial growth factor and basic fibroblast growth factor differentially modulate early postnatal coronary angiogenesis. Circ Res 2001; 88: 1135–41
Mattfeldt T, Mall G. Growth of capillaries and myocardial cells in the normal rat heart. J Mol Cell Cardiol 1987; 19: 1237–46
van Groningen JP, Wenink AC, Testers LH. Myocardial capillaries: Increase in number by splitting of existing vessels. Anat Embryol (Berl) 1991; 184: 65–70
Rakusan K, Poupa O. Changes in the diffusion distance in the rat heart muscle during development. Physiol Bohemoslov 1963; 12: 220–7
Rakusan K, Poupa O. The relationship between the capillaries and protein nitrogen in the myocardium of the rat during postnatal development. Physiol Bohemoslov 1965; 14: 320–3
Batra S, Rakusan K. Capillary network geometry during postnatal growth in rat hearts. Am J Physiol 1992; 262: H635–640
Roberts J, Wearn J. Quantitative changes in the capillary–muscle relationship in human hearts during normal growth and hypertrophy. Am Heart J 1941; 21: 617–33
Kurosawa S, Kurosawa H, Becker AE. The coronary arterioles in newborns, infants and children. A morphometric study of normal hearts and hearts with aortic atresia and complete transposition. Int J Cardiol 1986; 10: 43–56
Rakusan K, Cicutti N, Flanagan MF. Changes in the microvascular network during cardiac growth, development, and aging. Cell Mol Biol Res 1994; 40: 117–22
Wiest G, Gharehbaghi H, Amann K et al. Physiological growth of arteries in the rat heart parallels the growth of capillaries, but not of myocytes. J Mol Cell Cardiol 1992; 24: 1423–31
Ito T, Harada K, Tamura M, Takada G. In situ morphometric analysis of the coronary arterial growth in perinatal rats. Early Hum Dev 1998; 52: 21–6
Neufeld HN, Wagenvoort CA, Edwards JE. Coronary arteries in fetuses, infants, juveniles, and young adults. Lab Invest 1962; 11: 837–44
Dbaly J. Postnatal development of coronary arteries in the rat. J Anat Entwickl.-Gesch 1973; 141: 89–101
Hudlicka O, Brown MD. Postnatal growth of the heart and its blood vessels. J Vasc Res 1996; 33: 266–87
Mikawa T. Retroviral targeting of FGF and FGFR in cardiomyocytes and coronary vascular cells during heart development. Ann NY Acad Sci 1995; 752: 506–16
Fernandez B, Buehler A, Wolfram S et al. Transgenic myocardial overexpression of fibroblast growth factor-1 increases coronary artery density and branching. Circ Res 2000; 87: 207–13
Gibbons GH, Dzau VJ. Mechanisms of disease: The emerging concept of vascular remodeling. New Eng J Med 1994; 330: 1431–8
Sho E, Komatsu M, Sho M et al. High flow drives vascular endothelial cell proliferation during flow-induced arterial remodeling associated with the expression of vascular endothelial growth factor. Exp Mol Pathol 2003; 75: 1–11
Malek AM, Zhang J, Jiang J et al. Endothelin-1 gene suppression by shear stress: pharmacological evaluation of the role of tyrosine kinase, intracellular calcium, cytoskeleton, and mechanosensitive channels. J Mol Cell Cardiol 1999; 31: 387–99
Alon T, Hemo I, Itin A et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med 1995; 1: 1024–8
Upalakalin JN, Hemo I, Dehio C et al. Survival mechanisms of VEGF and PlGF during microvascular remodeling. Cold Spring Harb Symp Quant Biol 2002; 67: 181–7
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tomanek, R. Formation of the coronary vasculature during development. Angiogenesis 8, 273–284 (2005). https://doi.org/10.1007/s10456-005-9014-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-005-9014-9