Abstract
Background
Several Central and Eastern European (CEE) countries require cost-utility analyses (CUAs) to support reimbursement formulary listing. However, CUAs informed by local evidence are often unavailable, and the cost-effectiveness of the several currently reimbursed biologicals is unclear.
Aim
To estimate the cost-effectiveness as multiples of per capita GDP/quality adjusted life years (QALY) of four biologicals (infliximab, etanercept, adalimumab, golimumab) currently reimbursed in six CEE countries in six inflammatory rheumatoid and bowel disease conditions.
Methods
Systematic literature review of published cost-utility analyses in the selected conditions, using the United Kingdom (UK) as reference country and with study selection criteria set to optimize the transfer of results to the CEEs. Prices in each CEE country were pro-rated against UK prices using purchasing power parity (PPP)-adjusted per capita GDP, and local GDP per capita/QALY ratios estimated.
Results
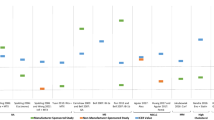
Central and Eastern European countries list prices were 144–333 % higher than pro rata prices. Out of 85 CUAs identified by previous systematic literature reviews, 15 were selected as a convenience sample for estimating the cost-effectiveness of biologicals in the CEE countries in terms of per capita GDP/QALY. Per capita GDP/QALY values varied from 0.42 to 6.4 across countries and conditions (Bulgaria: 0.97–6.38; Czech Republic: 0.42–2.76; Hungary: 0.54–3.54; Poland: 0.59–3.90; Romania: 0.77–5.07; Slovakia: 0.55–3.61).
Conclusion
While results must be interpreted with caution, calculating pro rata (cost-effective) prices and per capita GDP/QALY ratios based on CUAs can aid reimbursement decision-making in the absence of analyses using local data.
Similar content being viewed by others
Notes
Source: personal communication.
References
Gulácsi, L., Rotar, A.M., Niewada, M., Löblová, O., Rencz F., Petrova, G., Boncz, I., Klazinga, N.S.: Health technology assessment in Poland, the Czech Republic, Hungary, Romania and Bulgaria. Eur. J. Health Econ. This Supplement (2014). doi:10.1007/s10198-014-0590-8
Lopert, R., Lang, D.L., Hill, S.R., Henry, D.A.: Differential pricing of drugs: a role for cost-effectiveness analysis? Lancet 359, 2105–2107 (2002)
Lopert, R., Ruiz, F., Chalkidou, K.: Applying rapid ‘de-facto’ HTA in resource-limited settings: experience from Romania. Health Policy 112(3), 202–208 (2013)
Drummond, M., Barbieri, M., Cook, J., Glick, H.A., Lis, J., Malik, F., Reed, S.D., Rutten, F., Sculpher, M., Severens, J.: Transferability of economic evaluations across jurisdictions: ISPOR Good Research Practices Task Force report. Value Health 12(4), 409–418 (2009)
Péntek, M. (ed.): Systematic review and analysis of evidences on clinical efficacy and cost-effectiveness of biological drugs for the treatment of rheumatoid arthritis. Budapesti Corvinus Egyetem Egészségügyi Közgazdaságtan Tanszék, Budapest (2014). ISBN:978-963-503-575-5. Available at 19 Mar 2014. http://hecon.uni-corvinus.hu/download/konyv/Pentek_Biologicals.in.rheumatoid.arthritis_978_963_503_575_5_24.Febr.2014.pdf
Péntek, M.: Systematic review and analysis of evidences on clinical efficacy and cost-effectiveness of biological drugs for the treatment of Ankylosing Spondylitis. Budapesti Corvinus Egyetem Egészségügyi Közgazdaságtan Tanszék, Budapest (2013). ISBN: 978-963-503-561-8. Available at 19 Mar 2014. http://hecon.uni-corvinus.hu/download/konyv/Pentek_Biologicals.in.Ankylosing.Spondylitis_978_963_503_561_8_16.Dec.2013.pdf
Brodszky, V. (ed.): Systematic review and analysis of evidences on clinical efficacy and cost-effectiveness of biological drugs for the treatment of psoriatic arthritis. Budapesti Corvinus Egyetem Egészségügyi Közgazdaságtan Tanszék, Budapest (2014). ISBN:978-963-503-574-8. Available at 19 Mar 2014. http://hecon.uni-corvinus.hu/download/konyv/Brodszky_biologicals.in.Psoriatic.Arthritis_978_963_503_574_8_24.February.2014.pdf
Baji, P. (ed.): Systematic review and analysis of evidences on clinical efficacy and cost-effectiveness of biological drugs for the treatment of adult Crohn’s disease. Budapesti Corvinus Egyetem Egészségügyi Közgazdaságtan Tanszék, Budapest (2013). ISBN:978-963-503-558-8. Available at 19 Mar 2014. http://hecon.uni-corvinus.hu/download/konyv/Baji_Biologicals.in.Crohns.Disease_978_963_503_558_8_28.Nov.2013.pdf
Gulácsi, L.: Systematic review and analysis of evidences on clinical efficacy and cost-effectiveness of biological drugs for the treatment of adult Ulcerative Colitis. Budapesti Corvinus Egyetem Egészségügyi Közgazdaságtan Tanszék, Budapest (2013). ISBN: 978-963-503-557-1. Available at 19 Mar 2014. http://hecon.uni-corvinus.hu/download/konyv/Gulacsi_Biologicals.in.Ulcerative.Colitis_978_963_503_557_1_28.Nov.2013.pdf
Brodszky, V.: Systematic review and analysis of evidences on clinical efficacy and cost-effectiveness of biological drugs for the treatment of Psoriasis. Budapesti Corvinus Egyetem Egészségügyi Közgazdaságtan Tanszék, Budapest (2013). ISBN: 978-963-503-562-5. Available at 19 Mar 2014. http://hecon.uni-corvinus.hu/download/konyv/Brodszky_Biologicals.in.Psoriasis_978_963_503_562_5_16.Dec.2013.pdf
Péntek, M., Kobelt, G., Czirják, L., Szekanecz, Z., Poór, G., Rojkovich, B., Polgár, A., Genti, G., Kiss, C.G., Brodszky, V., Májer, I., Gulácsi, L.: Costs of rheumatoid arthritis in Hungary. J. Rheumatol. 34(6), 1437 (2007)
Brodszky, V., Bálint, P., Géher, P., Hodinka, L., Horváth, G., Koó, É., Péntek, M., Polgár, A., Seszták, M., Szántó, S., Ujfalussy, I., Gulácsi, L.: Disease burden of psoriatic arthritis compared to rheumatoid arthritis, Hungarian experiment. Rheumatol. Int. 30(2), 199–205 (2009)
Brodszky, V., Orlewska, E., Péntek, M., Kárpáti, K., Skoupá, J., Gulácsi, L.: Challenges in economic evaluation of new drugs: experience with rituximab in Hungary. Med. Sci. Monit. 16(1), SR1–SR5 (2010)
Brennan, A., Bansback, N., Reynolds, A., Conway, P.: Modelling the cost-effectiveness of etanercept in adults with rheumatoid arthritis in the UK. Rheumatology (Oxford). 43(1), 62–72 (2004)
Barbieri, M., Wong, J.B., Drummond, M.: The cost effectiveness of infliximab for severe treatment-resistant rheumatoid arthritis in the UK. Pharmacoeconomics. 23(6), 607-18 (2005)
Chen, Y.F., Jobanputra, P., Barton, P., Jowett, S., Bryan, S., Clark, W., Fry-Smith, A., Burls A.: A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess. 10(42), iii–iv, xi–xiii, 1–229 (2006)
Kobelt, G., Sobocki, P., Sieper, J., Braun, J.: Comparison of the cost-effectiveness of infliximab in the treatment of ankylosing spondylitis in the United Kingdom based on two different clinical trials. Int. J. Technol. Assess. Health Care 23, 368–375 (2007)
Ara, R.M., Reynolds, A.V., Conway, P.: The cost-effectiveness of etanercept in patients with severe ankylosing spondylitis in the UK. Rheumatology (Oxford) 46, 1338–1344 (2007)
Botteman, M.F., Hay, J.W., Luo, M.P., Curry, A.S., Wong, R.L., van Hout, B.A.: Cost effectiveness of adalimumab for the treatment of ankylosing spondylitis in the United Kingdom. Rheumatology (Oxford) 46(8), 1320–1328 (2007)
Riemsma, R., Joore, M., Van Asselt, T., Armstrong, N., Misso, K., Manning, N., Tomini, F., Kleijnen, J.: Golimumab for the treatment of ankylosing spondylitis: a single technology appraisal. Kleijnen Systematic Reviews Ltd., York (2011)
Bravo Vergel, Y., Hawkins, N.S., Claxton, K., Asseburg, C., Palmer, S., Woolacott, N., Bruce, I.N., Sculpher, M.J.: The cost-effectiveness of etanercept and infliximab for the treatment of patients with psoriatic arthritis. Rheumatology 46, 1729–1735 (2007)
Bojke, L., Epstein, D., Craig, D., Rodgers, M., Woolacott, N., Yang, H., Sculpher, M.: Modelling the cost-effectiveness of biologic treatments for psoriatic arthritis. Rheumatology 50(Suppl 4), iv39–iv47 (2011)
Yang, H., Craig, D., Epstein, D., Bojke, L., Light, K., Bruce, I.N., Sculpher, M., Woolacott, N.: Golimumab for the treatment of psoriatic arthritis: a NICE single technology appraisal. Pharmacoeconomics 30(4), 257–270 (2012)
Lindsay, J., Punekar, Y.S., Morris, J., Chung-Faye, G.: Health-economic analysis: cost-effectiveness of scheduled maintenance treatment with infliximab for Crohnʼs disease–modelling outcomes in active luminal and fistulizing disease in adults. Aliment. Pharmacol. Ther. 28, 76–87 (2008)
Bodger, K., Kikuchi, T., Hughes, D.: Cost-effectiveness of biological therapy for Crohnʼs disease: Markov cohort analyses incorporating United Kingdom patient-level cost data. Aliment. Pharmacol. Ther. 30, 265–274 (2009)
Loftus Jr, E.V., Johnson, S.J., Yu, A.P., Wu, E.Q., Chao, J., Mulani, P.M.: Cost-effectiveness of adalimumab for the maintenance of remission in patients with Crohnʼs disease. Eur. J. Gastroenterol. Hepatol. 21, 1302–1309 (2009)
Hyde, C., Bryan, S., Juarez-Garcia, A., Andronis, L., Fry-Smith, A.: Evidence review group report commissioned by the NHS R&D HTA programme on behalf of NICE infliximab for ulcerative colitis. West Midlands Health Technology Assessment Collaboration, Department of Public Health and Epidemiology, University of Birmingham (2007)
Sizto, S., Bansback, N., Feldman, S.R., Willian, M.K., Anis, A.H.: Economic evaluation of systemic therapies for moderate to severe psoriasis. Br. J. Dermatol. 160(6), 1264–1272 (2009)
Gaujoux-Viala, C., Fautrel, B.: Cost effectiveness of therapeutic interventions in ankylosis spondylitis. Pharmacoeconomics 30(12), 1145–1156 (2012)
de Pouvourville, G., Ulmann, P., Nixon, J., Boulenger, S., Glanville, J., Drummond, M.: The diffusion of health economics knowledge in Europe : the EURONHEED (European Network of Health Economics Evaluation Database) project. Pharmacoeconomics 23(2), 113–120 (2005)
Knies, S.: Transparency of transferability: diagnosing international aspects of economic evaluations of health care technologies. Universitaire Pers, Maastricht (2011)
Adapting existing HTAs from one country into other settings. EUnetHTA Adaptation Toolkit: Version 5. EUnetHTA (2011)
Reinhold, T., Brüggenjürgen, B., Schlander, M., Rosenfeld, S., Hessel, F., Willich, S.N.: Economic analysis based on multinational studies: methods for adapting findings to national contexts. J. Publ. Health. 18(4), 327–335 (2005)
Nixon, J., Rice, S., Drummond, M., Boulenger, S., Ulmann, P., de Pouvourville, G.: Guidelines for completing the EURONHEED transferability information checklists. Eur. J. Health Econ. 10(2), 157–165 (2008)
Gulácsi, L., Orlewska, E., Péntek, M.: Health economics and health technology assessment in Central and Eastern Europe: a dose of reality. Eur. J. Health Econ. 13(5), 525–531 (2012)
Boncz, I., Nagy, J., Sebestyen, A., Korosi, L.: Financing of health care services in Hungary. Eur. J. Health Econ. 5(3), 252–258 (2004)
Boncz, I., Sebestyen, A.: Financial deficits in the health services of the UK and Hungary. Lancet 368(9539), 917–918 (2006)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gulácsi, L., Rencz, F., Péntek, M. et al. Transferability of results of cost utility analyses for biologicals in inflammatory conditions for Central and Eastern European countries. Eur J Health Econ 15 (Suppl 1), 27–34 (2014). https://doi.org/10.1007/s10198-014-0591-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-014-0591-7