Abstract
Background
Although cancer-specific Health-Related Quality of Life (HRQOL) are commonly included in randomized clinical trials or other prospective non-randomized clinical studies, it is rare that preference-based instruments are used that allow the calculation of a utility weight suitable for estimating quality-adjusted life-years gained.
Objective
To develop a mapping algorithm to transform the EORTC QLQ-C30 questionnaire responses into EQ-5D derived utilities.
Study design
Retrospective data analysis of a multicentre, multicountry prospective clinical trial in breast cancer patients.
Methods
Regression analysis of individual pairs of EQ-5D and QLQ-C30 scores.
Results
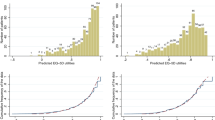
A model that explained 80% of the variance was developed to estimate EQ-5D Utilities from QLQ-C30 scores at individual level. From this reliable group level means and deviations can be derived.
Conclusions
Mapping from QLQ-C30 scores to EQ-5D-derived utilities when only QLQ-C30 data are available has been shown to be possible with good accuracy. Validation of the proposed algorithm in other external clinical datasets should be encouraged.


Similar content being viewed by others
Notes
Because we did not have access to the clinical data aside from the QOL data, it was not possible to further assess the responses by patients’ characteristics. However, in order to keep the practical application of the mapping algorithm across patient samples as general as possible, one does not want to include in the mapping equation any socio-demographic variables when considering mapping.
Utilities were transformed to Disutilities because this results in a right-skewed distribution bounded by zero on the left. This changes only the sign of the coefficients in the regressions.
The data provided to us were extracted from the cleaned and frozen clinical trial database from the EORTC, raising the suspicion of the existence of genuine outliers.
References
Efficace, F., Bottomley, A., Osoba, D., Gotay, C., Flechtner, H., D’haese, S., Zurlo, A.: Beyond the development of health-related quality-of-life (HRQOL) measures: a checklist for evaluating HRQOL outcomes in cancer clinical trials—does HRQOL evaluation in prostate cancer research inform clinical decision making? J. Clin. Oncol. 21(18), 3502–3511 (2003)
Fayers, P.M., Aaronson, N.K., Bjordal, K., Groenvold, M., Curran, D., Bottomley, A., on behalf of the EORTC Quality of Life Group: The EORTC QLQ-C30 scoring manual, 3rd edn. European Organisation for Research and Treatment of Cancer (EORTC), Brussels (2001)
Gotay, C.C., Kawamoto, C.T., Bottomley, A., Efficace, F.: The prognostic significance of patient-reported outcomes in cancer clinical trials. J. Clin. Oncol. 26(8), 1355–1363 (2008)
Therasse, P., Mauriac, L., Welnicka-Jaskiewicz, M., Bruning, P., Cufer, T., Bonnefoi, H., Tomiak, E., Pritchard, K.I., Hamilton, A., Piccart, M.J.: Final results of a randomized phase III trial comparing cyclophosphamide, epirubicin, and fluorouracil with a dose-Intensified epirubicin and cyclophosphamide–filgrastim as neoadjuvant treatment in locally advanced breast cancer: an EORTC-NCIC-SAKK multicenter study. J. Clin. Oncol. 21(5), 843–850 (2003)
EQ-5D Value sets: inventory, comparative review and user guide. In: Szende, A., Oppe, M., Devlin, N. (Eds.) EuroQol Group Monographs. Springer, Dordrecht (2007), ISBN 978-1402055102
Efficace, F., Therasse, P., Piccart, M.J., Coens, C., Van Steen, K., Welnicka-Jaskiewicz, M., Cufer, T., Dyczka, J., Lichinitser, M., Shepherd, L., de Haes, H., Sprangers, M.A., Bottomley, A.: Health-related quality of life parameters as prognostic factors in a nonmetastatic breast cancer population: an international multicenter study. J. Clin. Oncol. 22(16), 3381–3388 (2004)
Bottomley, A., Therasse, P., Piccart, M., Efficace, F., Coens, C., Gotay, C., Welnicka-Jaskiewicz, M., Dyczka, L.M.J., Cufer, T., Lichinitser, M.R., Schornagel, J.H., Bonnefoi, H., Shepherd, L., For the European Organisation for Researchand Treatment of Cancer Breast Cancer Group, the National Cancer Institute of Canada, Swiss Group for Clinical Cancer Research: Health-related quality of life in survivors of locally advanced breast cancer: an international randomised controlled phase III trial. Lancet Oncol 6, 287–294 (2005)
Dolan, P.: Modeling valuations for EuroQol health states. Med. Care 35(11), 1095–1108 (1997)
Dolan, P., Gudex, C., Kind, P., Williams, A.: The time trade-off method: results from a general population study. Health Econ. 5, 141–154 (1996)
Szende, A., Oppe, M., Devlin, N.: EQ-5D value sets, inventory, comparative review and user guide. Springer, Berlin (2006)
McKenzie, L., Van der Pol, M.: Mapping the EORTC QLQ C-30 onto the EQ-5D instrument: the potential to estimate QALY’s without generic preference data. Value Health 12(1), 167–171 (2009)
Gray, A.M., Rivero-Arias, O., Clarke, P.M.: Estimating the association between SF-12 responses and EQ-5D utility values by response mapping. Med. Decis. Mak. 26, 18–29 (2006)
Brazier, J.E., Yang, Y., Tsuchiya, A., Rowen, D.L.: A review of studies mapping (or cross walking) non-preference based measures of health to generic preference-based measures. Eur. J. Health Econ. (2009). doi:10.1007/s10198-009-0168-z
Peat, J., Barton, B.: Medical statistics: a guide to data analysis and critical appraisal, 1st edn., p. 150. BMJ Books, Oxford (2005)
Hardin, J.W., Hilbe, J.M.: Generalized estimation equations. Chapman & Hall/CRC, Boca Raton (2003)
Doornebosch, P.G., Tollenaar, R.A., Gosselink, M.P., Stassen, L.P., Dijkhuis, C.M., Schouten, W.R., van de Velde, C.J., de Graaf, E.J.: Quality of life after transanal endoscopic microsurgery and total Mesorectal excision in early rectal cancer. Colorectal Dis. 9(6), 553–558 (2007)
Gosselink, M.P., Busschbach, J.J., Dijkhuis, C.M., Stassen, L.P., Hop, W.C., Schouten, W.R.: Quality of life after total mesorectal excision for rectal cancer. Colorectal Dis. 8(1), 15–22 (2006)
Krabbe, P.F., Peerenboom, L., Langenhoff, B.S., Ruers, T.J.: Responsiveness of the generic EQ-5D summary measure compared to the disease-specific EORTC QLQ C-30. Qual. Life Res. 13(7), 1247–1253 (2004)
Krahn, M., Bremner, K.E., Tomlinson, G., Ritvo, P., Irvine, J., Naglie, G.: Responsiveness of disease-specific and generic utility instruments in prostate cancer patients. Qual. Life Res. 16(3), 509–522 (2007)
Wu, E.Q., Mulani, P., Farrell, M.H., Sleep, D.: Mapping FACT-P and EORTC QLQ-C30 to patient health status measured by EQ-5D in metastatic hormone-refractory prostate cancer patients. Value Health 10(5), 408–414 (2007)
Kind, P.: Measuring the quality of life on cancer: an index based on QLQ-C30 presentation, ASCO 2005 Annual Meeting, Category Health Services Research, abstract published in JCO, vol. 23, No. 16S, Part I of II, June 1 Supplement, 2005:6013
Kontodimopoulos, N., Aletras, V.H., Paliouras, D.: Mapping the cancer-specific EORTC QLQ-C30 to the preference-based EQ-5D, SF-6D and 15D instruments. Value Health, 12(8), 1151–1157 (2009)
McKenzie, L., Van der Pol, M.: Mapping the EORTC QLQ C-30 onto the EQ-5D instrument: the potential to estimate QALY’s without generic preference DATA. Value Health 12(1), 167–171 (2009)
Pickard, A.S., Shaw, J.W., Lin, H.W., Trask, P.C., Aaronson, N., Lee, T.A., Cella, D.: A patient-based utility measure of health for clinical trials of cancer therapy based on the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire. Value Health 12(6), 977–988 (2009)
Jang, R.W., Mittmann, N., Isogai, P., Bradbury, P.A., Leighl, N.B.: Derivation of utility values from EORTC QLQ-C30 values in lung cancer, ISPOR 2008, Toronto (Poster abstract PCN78). Value Health 11(3), A77 (2008)
Petrillo, J., Cairns, J.: Concerting condition-specific measures into preference-based outcomes for use in economic evaluation. Expert Rev. Pharmacoecon. Outcomes Res. 8(5), 453–461 (2008)
Bleeker, S.E., Moll, H.A., Steyerberg, E.W., Donders, A.R., Derksen-Lubsen, G., Grobbee, D.E., Moons, K.G.: External validation is necessary in prediction research: a clinical example. J. Clin. Epidemiol. 56(9), 826–832 (2003)
Steyerberg, E.W., Bleeker, S.E., Moll, H.A., Grobbee, D.E., Moons, K.G.: Internal and external validation of predictive models: a simulation study of bias and precision in small samples. J. Clin. Epidemiol. 56(5), 441–447 (2003)
Luo, N., Johnson, J.A., Shaw, J.W., Coons, S.J.: A comparison of the EQ-5D index scores derived from the US and UK population-based scoring functions. Med. Decis. Mak. 27, 321–326 (2007)
Chay, K.Y., Powell, J.L.: Semiparametric censored regression models. J. Econ. Perspect. 15(4), 29–42 (2001)
Sullivan, P., Ghushchyan, V.: Preference-based EQ-5D index scores for chronic conditions in the United States. Med. Decis. Mak. 26, 410–420 (2006)
Acknowledgments
This work was funded in part by an unrestricted grant from York Health Economics Consortium Ltd. The funding agreement ensured that the author’s independence in designing the study, interpreting the data, writing and publishing the report. We wish to thank Corinne O’Dowd for assisting in the cleaning and preparation of the data. We also are grateful to the EORTC Breast Cancer Group for providing access to the original trial data. The authors have no conflicts of interest that are directly relevant to the context of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Crott, R., Briggs, A. Mapping the QLQ-C30 quality of life cancer questionnaire to EQ-5D patient preferences. Eur J Health Econ 11, 427–434 (2010). https://doi.org/10.1007/s10198-010-0233-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-010-0233-7