Abstract
Background
We evaluated the efficacy and feasibility of paclitaxel in patients with gemcitabine-refractory pancreatic cancer. We also evaluated the correlation between tumor marker decline and response rate.
Methods
Thirty patients histologically diagnosed with pancreatic cancer who had undergone paclitaxel treatment as salvage chemotherapy were retrospectively analyzed. Paclitaxel treatment was performed with 80 mg/(m2 week) for 3 weeks followed by 1 week rest, and this was continued until failure. Tumor marker response was evaluated by measuring levels of carbohydrate antigen 19-9, carcinoembryonic antigen, and DUPAN-2 monthly.
Results
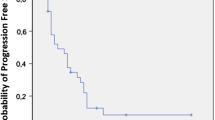
In total, 272 weekly paclitaxel treatments were performed. The median number of treatments per patient was 8 (range 1–22). The median overall survival from the start of paclitaxel treatment was 6.7 months (range 1.2–18.8). The response rate was 10% (3/30 patients) and the disease control rate was 46.7% (14/30 patients). Although grade 3 and 4 hematological and non-hematological toxicities were seen in 7 and 6 patients, respectively, adverse events were managed by conservative treatment. We found a significant correlation between the disease control rate and tumor marker decline within 2 months of paclitaxel treatment (P = 0.01). Patients with tumor marker decline tended to survive longer.
Conclusion
Weekly administration of paclitaxel in patients with gemcitabine-refractory pancreatic cancer seems to be well tolerated and can be effective. Paclitaxel treatment should be considered as salvage chemotherapy after gemcitabine failure in patients with good performance status.

Similar content being viewed by others
Abbreviations
- 5-FU:
-
5-Fluorouracil
- CA:
-
Carbohydrate antigen
- CEA:
-
Carcinoembryonic antigen
- CR:
-
Complete response
- FF:
-
5-Fluorouracil with folinic acid/leucovorin
- GEM:
-
Gemcitabine
- OFF:
-
5-Fluorouracil with folinic acid/leucovorin and oxaliplatin
- PD:
-
Progressive disease
- PR:
-
Partial response
- PTX:
-
Paclitaxel
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- SD:
-
Stable disease
- TM:
-
Tumor marker
- UFT:
-
Tegafur-uracil
References
Jemal A, Siegel R, Ward E et al (2009) Cancer statistics, 2009. CA Cancer J Clin 59:225–249
Matsuda T, Marugame T, Kamo K et al (2009) Cancer incidence and incidence rates in Japan in 2003: based on data from 13 population-based cancer registries in the Monitoring of Cancer Incidence in Japan (MCIJ) Project. Jpn J Clin Oncol 39:850–858
Burris HA 3rd, Moore MJ, Andersen J et al (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15:2403–2413
Matsuno S, Egawa S, Fukuyama S et al (2004) Pancreatic Cancer Registry in Japan: 20 years of experience. Pancreas 28:219–230
Ueno H, Kosuge T, Matsuyama Y et al (2009) A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer 101:908–915
Pelzer U, Kubica K, Stieler J et al (2008) A randomized trial in patients with gemcitabine refractory pancreatic cancer. Final results of the CONKO 003 study. J Clin Oncol 26:217 (abstract 4508)
Rowinsky EK (1997) The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu Rev Med 48:353–374
Oettle H, Arnold D, Esser M et al (2000) Paclitaxel as weekly second-line therapy in patients with advanced pancreatic carcinoma. Anticancer Drugs 11:635–638
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Burris HA 3rd (2005) Recent updates on the role of chemotherapy in pancreatic cancer. Semin Oncol 32:S1–S3
Kim YJ, Bang S, Park JY et al (2009) Phase II study of 5-fluorouracil and paclitaxel in patients with gemcitabine-refractory pancreatic cancer. Cancer Chemother Pharmacol 63:529–533
Nakachi K, Furuse J, Ishii H et al (2007) Prognostic factors in patients with gemcitabine-refractory pancreatic cancer. Jpn J Clin Oncol 37:114–120
Ikeda M, Okada S, Tokuuye K et al (2001) Prognostic factors in patients with locally advanced pancreatic carcinoma receiving chemoradiotherapy. Cancer 91:490–495
Krishnan S, Rana V, Janjan NA et al (2006) Prognostic factors in patients with unresectable locally advanced pancreatic adenocarcinoma treated with chemoradiation. Cancer 107:2589–2596
Ueno H, Okada S, Okusaka T et al (2000) Prognostic factors in patients with metastatic pancreatic adenocarcinoma receiving systemic chemotherapy. Oncology 59:296–301
Ko AH, Hwang J, Venook AP et al (2005) Serum CA 19-9 response as a surrogate for clinical outcome in patients receiving fixed-dose rate gemcitabine for advanced pancreatic cancer. Br J Cancer 93:195–199
Stemmler J, Stieber P, Szymala AM et al (2003) Are serial CA 19-9 kinetics helpful in predicting survival in patients with advanced or metastatic pancreatic cancer treated with gemcitabine and cisplatin? Onkologie 26:462–467
Wong D, Ko AH, Hwang J et al (2008) Serum CA 19-9 decline compared to radiographic response as a surrogate for clinical outcomes in patients with metastatic pancreatic cancer receiving chemotherapy. Pancreas 37:269–274
Halm U, Schumann T, Schiefke I et al (2000) Decrease of CA 19-9 during chemotherapy with gemcitabine predicts survival time in patients with advanced pancreatic cancer. Br J Cancer 82:1013–1016
Ziske C, Schlie C, Gorschluter M et al (2003) Prognostic value of CA 19-9 levels in patients with inoperable adenocarcinoma of the pancreas treated with gemcitabine. Br J Cancer 89:1413–1417
Maisey NR, Norman AR, Hill A et al (2005) CA 19-9 as a prognostic factor in inoperable pancreatic cancer: the implication for clinical trials. Br J Cancer 93:740–743
Berlin JD, Catalano P, Thomas JP et al (2002) Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol 20:3270–3275
Louvet C, Labianca R, Hammel P et al (2005) Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol 23:3509–3516
Poplin E, Feng Y, Berlin J et al (2009) Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol 27:3778–3785
Agheli A, Park SC, Huang CJ et al (2009) Apparent beneficial effects by nab-paclitaxel in the treatment of refractory metastatic ovarian carcinoma. Anticancer Drugs 20:525–526
Gradishar WJ, Krasnojon D, Cheporov S et al (2009) Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol 27:3611–3619
Rizvi NA, Riely GJ, Azzoli CG et al (2008) Phase I/II trial of weekly intravenous 130-nm albumin-bound paclitaxel as initial chemotherapy in patients with stage IV non-small-cell lung cancer. J Clin Oncol 26:639–643
Robinson DM, Keating GM (2006) Albumin-bound paclitaxel: in metastatic breast cancer. Drugs 66:941–948
Acknowledgment
This work supported in part by Grants-in-Aid for Scientific Research (C) 21591766 from Japan society for the Promotion of Science.
Conflict of interest
No author has any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Maeda, S., Motoi, F., Onogawa, T. et al. Paclitaxel as second-line chemotherapy in patients with gemcitabine-refractory pancreatic cancer: a retrospective study. Int J Clin Oncol 16, 539–545 (2011). https://doi.org/10.1007/s10147-011-0220-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-011-0220-8