Abstract
Purpose
Patient-reported outcomes (PROs) are an increasingly popular tool to optimize care and bridge the gap between patient experience and clinician understanding. The aim of this review was to identify mechanisms through which PROs facilitate patient-clinician communication in the adult oncology population.
Methods
We conducted a systematic review of the published literature using the following data sources: MEDLINE, EMBASE, CINAHL, PsycINFO, Cab Direct, and CDSR. Studies included in this review reported on the outcomes of PRO use, used PROs as an intervention and not as a study outcome measurement tool, included cancer patients or survivors as study participants, and analyzed patient-clinician communication.
Results
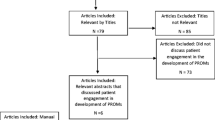
We identified 610 unique records, of which 43 publications met the inclusion and exclusion criteria. Synthesis of the reviewed studies provided evidence of the usefulness of PROs in facilitating patient-clinician communication on a variety of topics. We identified mechanisms though which PROs influenced patient-clinician communication to include increasing symptom awareness, prompting discussion, streamlining consultations, and facilitating inter-professional communication. Barriers to PRO use in communication improvement include technical problems impeding its administration and completion, compliance issues due to lack of incentive or forgetfulness, and use of PROs that do not appropriately assess issues relevant to the patient. Facilitators include increased education on PRO use, using PRO tools that patients find more acceptable, and providing patient data summaries in an easily accessible format for clinicians.
Conclusions
Our review suggests that PROs facilitate patient-clinician communication through various mechanisms that could perhaps contribute to improvements in symptom management and survival. The impact of PROs on clinical outcomes, however, remains poorly studied.


Similar content being viewed by others
References
Patient reported outcomes (PROs) in performance measurement (2012) Washington DC: National Quality Forum. Available online at https://www.qualityforum.org/Publications/2012/12/Patient-Reported_Outcomes_in_Performance_Measurement.aspx
Bennett AV, Jensen RE, Basch E (2012) Electronic patient-reported outcome systems in oncology clinical practice. CA Cancer J Clin 62:337–347. https://doi.org/10.3322/caac.21150
Chen J, Ou L, Hollis SJ (2013) A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res 13:211. https://doi.org/10.1186/1472-6963-13-211
Kotronoulas G, Kearney N, Maguire R, Harrow A, Di Domenico D, Croy S et al (2014) What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol 32:1480–1501. https://doi.org/10.1200/JCO.2013.53.5948
Marshall S, Haywood K, Fitzpatrick R (2006) Impact of patient-reported outcome measures on routine practice: a structured review. J Eval Clin Pract 12:559–568. https://doi.org/10.1111/j.1365-2753.2006.00650.x
Wolfe J, Orellana L, Cook EF, Ullrich C, Kang T, Geyer JR et al (2014) Improving the care of children with advanced cancer by using an electronic patient-reported feedback intervention: results from the PediQUEST randomized controlled trial. J Clin Oncol 32:1119–1126. https://doi.org/10.1200/JCO.2013.51.5981
Howell D, Molloy S, Wilkinson K, Green E, Orchard K, Wang K et al (2015) Patient-reported outcomes in routine cancer clinical practice: a scoping review of use, impact on health outcomes, and implementation factors. Ann Oncol 26:1846–1858. https://doi.org/10.1093/annonc/mdv181
Greenhalgh J, Abhyankar P, McCluskey S, Takeuchi E, Velikova G (2013) How do doctors refer to patient-reported outcome measures (PROMS) in oncology consultations? Qual Life Res 22:939–950. https://doi.org/10.1007/s11136-012-0218-3
Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P et al (2016) Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 34:557–565. https://doi.org/10.1200/JCO.2015.63.0830
Snyder CF, Jensen RE, Geller G, Carducci MA, Wu AW (2010) Relevant content for a patient-reported outcomes questionnaire for use in oncology clinical practice: putting doctors and patients on the same page. Qual Life Res 19:1045–1055. https://doi.org/10.1007/s11136-010-9655-z
Kuijpers W, Groen WG, Loos R, Oldenburg HSA, Wouters MWJM, Aaronson NK et al (2015) An interactive portal to empower cancer survivors: a qualitative study on user expectations. Support Care Cancer 23:2535–2542. https://doi.org/10.1007/s00520-015-2605-0
Greenhalgh J, Long AF, Flynn R (2005) The use of patient reported outcome measures in routine clinical practice: lack of impact or lack of theory? Soc Sci Med 60:833–843. https://doi.org/10.1016/j.socscimed.2004.06.022
Wong G (2012) The internet in medical education: a worked example of a realist review, in synthesizing qualitative research: choosing the right approach. In: Hannes K, Lockwood C (eds) Synthesizing qualitative resarch: choosing the right approach. Wiley, Chichester, p 83
McCormack B, Rycroft-Malone J, Decorby K, Hutchinson AM, Bucknall T, Kent B et al (2013) A realist review of interventions and strategies to promote evidence-informed healthcare: a focus on change agency. Implement Sci 8:107. https://doi.org/10.1186/1748-5908-8-107
Pearson M, Brand SL, Quinn C, Shaw J, Maguire M, Michie S et al (2015) Using realist review to inform intervention development: methodological illustration and conceptual platform for collaborative care in offender mental health. Implement Sci 10:134. https://doi.org/10.1186/s13012-015-0321-2
Greenhalgh J, Pawson R, Wright J, Black N, Valderas JM, Meads D et al (2014) Functionality and feedback: a protocol for a realist synthesis of the collation, interpretation and utilisation of PROMs data to improve patient care. BMJ Open 4:e005601. https://doi.org/10.3310/hsdr05020
Rycroft-Malone J, McCormack B, Hutchinson AM, DeCorby K, Bucknall TK, Kent B et al (2012) Realist synthesis: illustrating the method for implementation research. Implement Sci 7:33. https://doi.org/10.1186/1748-5908-7-33
Greenhalgh T, Wong G, Westhorp G, Pawson R (2011) Protocol—realist and meta-narrative evidence synthesis: evolving standards (RAMESES). BMC Med Res Methodol 11:115. https://doi.org/10.1186/1471-2288-11-115
Higgins JPT, Green S (editors) (2008) Cochrane handbook for systematic reviews of interventions: Cochrane book series. The Cochrane Collaboration https://doi.org/10.1002/9780470712184
Brettle AJ, Long AF, Grant MJ, Greenhalgh J (1998) Searching for information on outcomes: do you need to be comprehensive? Quality in health care 7:163–167. https://doi.org/10.1136/qshc.7.3.163
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700. https://doi.org/10.1136/bmj.b2700
Higgins JPT, Green S (editors) (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane collaboration available from www.handbook.cochrane.org
Berry DL, Blumenstein BA, Halpenny B, Wolpin S, Fann JR, Austin-Seymour M et al (2011) Enhancing patient-provider communication with the electronic self-report assessment for cancer: a randomized trial. J Clin Oncol 29:1029–1035. https://doi.org/10.1200/JCO.2010.30.3909
Halkett G (2010) Trialling computer touch-screen technology to assess psychological distress in patients with gynaecological cancer. Australas Med J 3:781–785. https://doi.org/10.4066/AMJ.2010.446
Mullen KH, Berry DL, Zierler BK (2004) Computerized symptom and quality-of-life assessment for patients with cancer part II: acceptability and usability. Oncol Nurs Forum 31:E84–E89. https://doi.org/10.1188/04.ONF.E75-E83
Girgis A, Breen S, Stacey F, Lecathelinais C (2009) Impact of two supportive care interventions on anxiety, depression, quality of life, and unmet needs in patients with nonlocalized breast and colorectal cancers. J Clin Oncol 27:6180–6190. https://doi.org/10.1200/JCO.2009.22.8718
Bainbridge D, Seow H, Sussman J, Pond G, Martelli-Reid L, Herbert C et al (2011) Multidisciplinary health care professionals’ perceptions of the use and utility of a symptom assessment system for oncology patients. J Clin Oncol 7:19–23. https://doi.org/10.1200/JOP.2010.000015
Hilarius DL, Kloeg PH, Gundy CM, Aaronson NK (2008) Use of health-related quality-of-life assessments in daily clinical oncology nursing practice: a community hospital-based intervention study. Cancer 113:628–637. https://doi.org/10.1002/cncr.23623
Detmar SB, Muller MJ, Schornagel JH, Wever LDV, Aaronson NK (2002) Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA 8:3027–3034. https://doi.org/10.1001/jama.288.23.3027
Velikova G, Keding A, Harley C, Cocks K, Booth L, Smith AB et al (2010) Patients report improvements in continuity of care when quality of life assessments are used routinely in oncology practice: secondary outcomes of a randomised controlled trial. Eur J Cancer 46:2381–2388. https://doi.org/10.1016/j.ejca.2010.04.030
Hartzler AL, Izard JP, Dalkin BL, Mikles SP, Gore JL (2016) Design and feasibility of integrating personalized PRO dashboards into prostate cancer care. J Am Med Inform Assoc 23:38–47. https://doi.org/10.1093/jamia/ocv101
Detmar SB, Aaronson NK (1998) Quality of life assessment in daily clinical oncology practice: a feasibility study. Eur J Cancer 34:1181–1186. https://doi.org/10.1016/S0959-8049(98)00018-5
Snyder CF, Blackford AL, Wolff AC, Carducci MA, Herman JM, Wu AW et al (2013) Feasibility and value of PatientViewpoint: a web system for patient-reported outcomes assessment in clinical practice. Psychooncology 22:895–901. https://doi.org/10.1002/pon.3087
Velikova G, Booth L, Smith AB, Brown PM, Lynch P, Brown JM et al (2004) Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol 22:714–724. https://doi.org/10.1200/JCO.2004.06.078
Takeuchi EE, Keding A, Awad N, Hofmann U, Campbell LJ, Selby PJ et al (2011) Impact of patient-reported outcomes in oncology: a longitudinal analysis of patient-physician communication. J Clin Oncol 29:2910–2917. https://doi.org/10.1200/JCO.2010.32.2453
Fromme EK, Holliday EB, Nail LM, Lyons KS, Hribar MR, Thomas CR Jr (2016) Computerized patient-reported symptom assessment in radiotherapy: a pilot randomized, controlled trial. Support Care Cancer 24:1897–1906. https://doi.org/10.1007/s00520-015-2983-3
Mark TL, Fortner B, Johnson G (2008) Evaluation of a tablet PC technology to screen and educate oncology patients. Support Care Cancer 16:371–378. https://doi.org/10.1007/s00520-007-0312-1
Braeken APBM, Kempen GIJM, Eekers D, van Gils FCJM, Houben RMA, Lechner L (2011) The usefulness and feasibility of a screening instrument to identify psychosocial problems in patients receiving curative radiotherapy: a process evaluation. BMC Cancer 11:479. https://doi.org/10.1186/1471-2407-11-479
Dinkel A, Berg P, Pirker C, Geinitz H, Sehlen S, Emrich M et al (2010) Routine psychosocial distress screening in radiotherapy: implementation and evaluation of a computerised procedure. Br J Cancer 103:1489–1495. https://doi.org/10.1038/sj.bjc.6605930
Lim S, Han H, Lee K, Lee S, Kim J, Yun J et al (2015) A satisfaction survey on cancer pain management using a self-reporting pain assessment tool. J Palliat Med 18:225–231. https://doi.org/10.1089/jpm.2012.0183
Kornblith AB, Dowell JM, Herndon JE 2nd, Engelman BJ, Bauer-Wu S, Small EJ et al (2006) Telephone monitoring of distress in patients aged 65 years or older with advanced stage cancer: a cancer and leukemia group B study. Cancer 107:2706–2714. https://doi.org/10.1002/cncr.22296
Weaver A, Young AM, Rowntree J, Townsend N, Pearson S, Smith J et al (2007) Application of mobile phone technology for managing chemotherapy-associated side-effects. Ann Oncology 18:1887–1892. https://doi.org/10.1093/annonc/mdm354
Lynch J, Goodhart F, Saunders Y, O’Connor SJ (2011) Screening for psychological distress in patients with lung cancer: results of a clinical audit evaluating the use of the patient distress thermometer. Support Care Cancer 19:193–202. https://doi.org/10.1007/s00520-009-0799-8
Maguire R, Ream E, Richardson A, Connaghan J, Johnston B, Kotronoulas G et al (2015) Development of a novel remote patient monitoring system: the advanced symptom management system for radiotherapy to improve the symptom experience of patients with lung cancer receiving radiotherapy. Cancer Nurs 38:E37–E47. https://doi.org/10.1097/NCC.0000000000000150
Mooney KH, Beck SL, Friedman RH, Farzanfar R (2002) Telephone-linked care for cancer symptom monitoring: a pilot study. Cancer Pract 10:147–154. https://doi.org/10.1046/j.1523-5394.2002.103006.x
Basch E, Artz D, Dulko D, Scher K, Sabbatini P, Hensley M et al (2005) Patient online self-reporting of toxicity symptoms during chemotherapy. J Clin Oncol 23:3552–3561. https://doi.org/10.1200/JCO.2005.04.275
Basch E, Iasonos A, Barz A, Culkin A, Kris MG, Artz D et al (2007) Long-term toxicity monitoring via electronic patient-reported outcomes in patients receiving chemotherapy. J Clin Oncol 25:5374–5380. https://doi.org/10.1200/JCO.2007.11.2243
Rogers SN, Lowe D (2014) An evaluation of the head and neck cancer patient concerns inventory across the Merseyside and Cheshire network. Br J Oral Maxillofac Surg 52:615–623. https://doi.org/10.1016/j.bjoms.2014.04.011
Hopwood P (1998) Living with advanced breast cancer: development and application of a clinical checklist for patients on endocrine therapy. Breast 7:14–21. https://doi.org/10.1016/S0960-9776(98)90046-7
Wilkie DJ, Judge MKM, Berry DL, Dell J, Zong S, Gilespie R (2003) Usability of a computerized PAINReportIt in the general public with pain and people with cancer pain. J Pain Symptom Manag 25:213–224. https://doi.org/10.1016/S0885-3924(02)00638-3
Patel RA, Klasnja P, Hartzler A, Unruh KT, Pratt W (2012) Probing the benefits of real-time tracking during cancer care. AMIA Ann Symp Proc 2012:1340
Mooney KH, Beck SL, Friedman RH, Farzanfar R, Wong B (2014) Automated monitoring of symptoms during ambulatory chemotherapy and oncology providers’ use of the information: a randomized controlled clinical trial. Support Care Cancer 22:2343–2350. https://doi.org/10.1007/s00520-014-2216-1
Taenzer P, Bultz BD, Carlson LE, Speca M, DeGagne T, Olson K et al (2000) Impact of computerized quality of life screening on physician behaviour and patient satisfaction in lung cancer outpatients. Psychooncology 9:203–213. https://doi.org/10.1002/1099-1611(200005/06)9:3<203::AID-PON453>3.0.CO;2-Y
Wagner LI, Schink J, Bass M, Patel S, Diaz MV, Rothrock N et al (2015) Bringing PROMIS to practice: brief and precise symptom screening in ambulatory cancer care: PROMIS symptom assessment in cancer care. Cancer 121:927–934. https://doi.org/10.1002/cncr.29104
Berry DL, Hong F, Halpenny B, Partridge A, Fox E, Fann JR et al (2014) The electronic self report assessment and intervention for cancer: promoting patient verbal reporting of symptom and quality of life issues in a randomized controlled trial. BMC Cancer 14:513. https://doi.org/10.1186/1471-2407-14-513
Davis KM, Dawson D, Kelly S, Red S, Penek S, Lynch J et al (2013) Monitoring of health-related quality of life and symptoms in prostate cancer survivors: a randomized trial. J Support Oncol 11:174
Mehanna HM, Morton RP (2006) Patients views on the utility of quality of life questionnaires in head and neck cancer: a randomised trial. Clin Otolaryngol 31:310–316. https://doi.org/10.1111/j.1749-4486.2006.01256.x
Boyes A, Newell S, Girgis A, McElduff P, Sanson-Fisher RR (2006) Does routine assessment and real-time feedback improve cancer patients’ psychosocial well-being? Eur J Cancer Care 15:163–171. https://doi.org/10.1111/j.1365-2354.2005.00633.x
Hoekstra J, de Vos R, van Duijn NP, Schadé E, Bindels PJE (2006) Using the symptom monitor in a randomized controlled trial: the effect on symptom prevalence and severity. J Pain Symptom Manag 31:22–30. https://doi.org/10.1016/j.jpainsymman.2005.06.014
Rosenbloom SK, Victorson DE, Hahn EA, Peterman AH, Cella D (2007) Assessment is not enough: a randomized controlled trial of the effects of HRQL assessment on quality of life and satisfaction in oncology clinical practice. Psychooncology 16:1069–1079. https://doi.org/10.1002/pon.1184
Dawes AJ, Reardon S, Chen VL, Kaiser W, Russell MM, Ko CY et al (2015) Wireless technology to track surgical patients after discharge: a pilot study. Am Surg 81:1061
Wysham NG, Wolf SP, Samsa G, Abernethy AP, LeBlanc TW (2017) Integration of electronic patient-reported outcomes into routine cancer care: an analysis of factors affecting data completeness. J Clin Oncol Clinical Cancer Informatics 1:1–10. https://doi.org/10.1200/cci.16.00043
Ganz PA, Gotay CC (2007) Use of patient-reported outcomes in phase III cancer treatment trials: lessons learned and future directions. J Clin Oncol 25:5063–5069. https://doi.org/10.1200/jco.2007.11.0197
Acknowledgements
We thank Katherine Miller (UBC), for her contributions to creating and editing the search strategy used in this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Appendix. Search strategy
Appendix. Search strategy
CDSR was searched using the OvidSP interface on August 8, 2016, for publications prior to July 2016, with an adult population and published in English.
1 cancer*.ab,ti.
2 tumo?r.ab,ti.
3 neoplasm*.ab,ti.
4 oncolog*.ab,ti.
5 carcinoma*.ab,ti.
6 lymphoma*.ab,ti.
7 sarcoma*.ab,ti.
8 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7.
9 PROM.
10 PROMIS.
11 PROMs.
12 patient reported outcome*.
13 (self or patient*) adj1 (questionnaire* or report*).
14 9 OR 10 OR 11 OR 12 OR 13.
15 communicat*.
16 (patient* adj6 (provider* or physician* or clinician* or nurse* or oncologist*) adj6 (interaction* or communication* or discuss*)).mp.
17 15 OR 16.
#18 8 and 14 and 17.
CINAHL was searched using the EBSCO interface on July 14, 2016, for publications prior to July 2016.
1 cancer*.
2 tumo?r.
3 carcinoma*.
4 lymphoma*.
5 sarcoma*.
6 (MH “Neoplasms+”).
7 (MH “Oncology+”).
8 (MH “Cancer Patients”).
9 (MH “Cancer Survivors”).
10 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9.
11 PROM.
12 PROMIS.
13 Patient reported outcome.
14 self N1 report N1 questionnaire.
15 (MH “Outcome Assessment”).
16 (MM “Questionnaires+”).
17 (MM “Scales”).
18 (MM “Severity of Illness Indices”).
19 (MM “Short Form-36 Health Survey”).
20 (MM “Self Report”).
21 S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21.
22 communication.
23 (MH “Communication+”).
24 (MH “Physician-Patient Relations”) OR (MH “Nurse-Patient Relations”).
25 (patient* N2 (provider* or physician* or clinician* or nurse* or oncologist*) N2 (interaction* or communication* or discuss*)).
26 S22 OR S23 OR S24 OR S25.
27 S10 AND S21 AND S26.
28 Limit 27 by english, all adult.
EMBASE was searched using the OvidSp interface on July 14, 2016, for publications prior to July 2016.
1 cancer*.ab,ti.
2 tumo?r.ab,ti.
3 carcinoma*.ab,ti.
4 lymphoma*.ab,ti.
5 sarcoma*.ab,ti.
6 neoplasm*.ab,ti.
7 oncolog*.ab,ti.
8 Oncology/.
9 Cancer Patient/.
10 Neoplasm/.
11 or/ 1–10.
12 PROM.
13 PROMs.
14 PROMIS.
15 patient reported outcome*.
16 patient* outcome* measure*.
17 self report adj1 (questionnaire or measure).
18 Health Assessment/.
19 Exp *Questionnaire/.
20 *Self Report/.
21 Exp *Health Survey/.
22 or/ 12–21.
23 (patient* adj2 (provider* or physician* or clinician* or nurse* or oncologist*) adj2 (interaction* or communication* or discuss*)).mp.
24 Nurse Patient Relationship/.
25 Doctor Patient Relation/.
26 Interpersonal Communication/.
27 communication.mp.
28 or/ 23–28.
29 11 and 22 and 28
30 limit 29 to (adult <18 to 64 years > or aged <65+ years>).
31 limit 30 to (human and english language).
MEDLINE was searched using the OvidSp interface on July 14, 2016, for publications prior to July 2016.
1 cancer*.ab,ti.
2 tumo?r.ab,ti.
3 carcinoma*.ab,ti.
4 lymphoma*.ab,ti.
5 sarcoma*.ab,ti.
6 neoplasm*.ab,ti.
7 oncolog*.ab,ti.
8 Exp Neoplasms/.
9 Exp Medical Oncology/.
10 or/ 1–9.
11 PROM.
12 PROMs.
13 PROMIS.
14 patient reported outcome*.
15 patient* outcome* measure*.
16 self report adj1 (questionnaire or measure).
17 Exp *Health Status Indicators/.
18 *Questionnaires/.
19 *Psychometrics/.
20 Self Report/.
21 Health Surveys/.
22 or/ 11–21.
23 Communication/ or communication.mp.
24 (patient* adj2 (provider* or physician* or clinician* or nurse* or oncologist*) adj2 (interaction* or communication* or discuss*)).mp.
25 Exp Professional-Patient Relations.
26 or/ 23–25.
27 and/ 10,22,26.
28 limit 27 to english.
29 limit 28 to human.
30 limit 29 to “all adult (19 plus years)”.
Rights and permissions
About this article
Cite this article
Yang, L.Y., Manhas, D.S., Howard, A.F. et al. Patient-reported outcome use in oncology: a systematic review of the impact on patient-clinician communication. Support Care Cancer 26, 41–60 (2018). https://doi.org/10.1007/s00520-017-3865-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-017-3865-7