Abstract
Purpose
This study examines posttraumatic growth (PTG) among adolescents and young adults (AYAs) with cancer, as well as its correlates and trajectories over time. The study also explores the buffering role of PTG on the associations between posttraumatic stress (PTS), health-related quality of life (HRQoL), and psychological distress.
Methods
A multicenter, longitudinal, prospective study was conducted among AYA cancer patients aged 14–39 years. One hundred sixty-nine patients completed a self-report measure of PTG (PTGI) and PTS (PDS) 6, 12, and 24 months after baseline (within the first 4 months of diagnosis). At 24-month follow-up, HRQoL (SF-36) and psychological distress (BSI-18) were also assessed.
Results
Among participants, 14% showed increasing PTG, 45% remained at a stable high PTG level, 14% showed decreasing PTG, and 27% remained at a stable low PTG level. AYAs who remained high on PTG were more often younger, female, and received chemotherapy. PTG level at 6-month follow-up was predictive of mental HRQoL (β = 0.19; p = 0.026) and psychological distress (β = −0.14; p = 0.043) at 24-month follow-up when corrected for PTS and sociodemographic and clinical covariates. No relationship between PTG and physical HRQoL was found. The interactive effects of PTS and PTG on outcomes were not significant, indicating that buffering did not take place.
Conclusion
This study indicates that PTG is dynamic and predicts mental well-being outcomes but does not buffer the effects of PTS. Psychosocial interventions should focus on promoting PTG and reducing PTS in order to promote the adjustment of AYAs diagnosed with cancer.
Similar content being viewed by others
Introduction
The common trend of combining the adolescents and young adults (AYA) with cancer, defined here as persons diagnosed between 15 and 39 years of age at time of diagnosis [1], with either pediatric or older adult cancer populations in psychosocial research has created a gap in understanding the cancer experience of AYAs [2]. Adolescence and young adulthood are life stages characterized by increased vulnerability to stress as age-related developmental tasks need to be achieved [3, 4], such as completing school and becoming independent from parents for adolescents and starting a family and getting a mortgage for young adults. A cancer diagnosis challenges AYAs’ abilities to achieve these developmental milestones [5] and can compromise AYAs’ adjustment and health-related quality of life (HRQoL) [6].
Much of today’s psychosocial oncology research harkens to Selye’s theoretical stress and coping models, in which human emotional response to upsetting or traumatic events or conditions may be experienced as “distress” (negative) or “eustress” (positive) [7]. Subsequent applications of stress coping theory to investigations of patients’ psychosocial adaptation to cancer suggest that it is theoretically possible to experience both positive and negative responses to cancer simultaneously. Tedeschi and Calhoun have conceptualized positive adaptation to traumatic life events, including serious illness, as posttraumatic growth (PTG) [8, 9]. Positive changes reported by some cancer survivors have included a greater sense of closeness to others, higher appreciation of everyday life, recognition of new possibilities in life, a sense of personal strength, and deeper spirituality [10]. In a semi-structured interview study of AYAs’ diagnosed and treated for cancer [11], two recurrent themes emerged: (1) loss of control resulting in a sense of frustration and anger and (2) benefit finding such as improved personal attributes and strengthened relationships. All but one participant reported that they experienced at least one positive aspect of being treated for cancer. Quantitative research examining the prevalence of PTG in AYA cancer populations is scarce but suggests that most AYA cancer patients report some PTG [12–16].
There is controversy in the literature about how to conceptualize PTG: as an outcome of coping with a stressful life event similar to well-being [17] or as a process of coping with adversity, which may lead to positive adjustment [18] but is conceptually distinct from it. Findings regarding the relationships between PTG and well-being in cancer patients have been inconsistent; some studies have found positive (curvilinear) correlations with adjustment outcomes, while others find negative relations or even null findings [10, 19]. Several hypotheses may explain these inconsistencies including varying PTG levels over time within individuals. However, another possible explanation for these inconsistencies is that PTG may influence well-being not only as a direct effect but also through a stress-buffering mechanism. The ability to identify positive aspects of their cancer experience may help to protect patients from the adverse effects of cancer and its treatment. For example, a study of breast cancer patients showed that PTG moderated relationships between posttraumatic stress (PTS) and both psychological distress and HRQoL [20]. These authors suggested that PTG may help patients to reinterpret and accept the threatening aspects of the experience, thus restoring a sense of security and lessening the impact of PTS symptoms on well-being.
Prior investigations indicated that 39–48% of AYA cancer patients report PTS symptoms [21, 22], and that these symptoms negatively impact HRQoL and psychological distress [23]. It is therefore important to know if PTG is simply an outcome or if it can also act as a stress-buffering process. Considering the gaps in our understanding of PTG among AYAs with cancer, the aims of this study are to (1) examine trajectories of PTG over time, (2) identify the correlates that distinguish PTG trajectories, (3) determine the predictive value of PTG on HRQoL and psychological distress, and (4) examine the buffering role of PTG on the association between PTS and HRQoL and psychological distress. Findings will help health care providers readily identify AYAs who may benefit from targeted interventions that not only aim to reduce psychological distress but also promote positive adaptation and growth.
Methods
Design, procedure, and patients
A prospective, longitudinal, multisite study was conducted to examine HRQoL outcomes over 2 years in AYA patients recently diagnosed with cancer. Baseline data were collected within the first 4 months of diagnosis and subsequently at 6, 12, and 24 months after the baseline survey. The current study focuses on assessments of PTG administered at the three follow-up points. Eligibility criteria included patients aged 14 (and anticipated to turn 15 years old during treatment) through 39 years, with a first diagnosis of any form of invasive cancer, and the ability to read and understand English or Spanish. Participating institutions included three pediatric care institutions and two university-affiliated adult care medical institutions. Research staff at each institution monitored clinic rosters and subsequently identified and approached a total of 286 eligible patients over a 25-month period. Fifty-eight patients did not provide consent, either because they refused participation or because physicians denied access to patients who were too sick to participate. An additional 12 AYAs did not return a completed survey after providing consent, and one died before completing the baseline survey. Overall participation rate was 75% (n = 215). Institutional Review Board approval was obtained from each participating site and coordinating center. Informed consent and/or assent was obtained from patients and parents.
Measures
Posttraumatic growth was measured by the Posttraumatic Growth Inventory (PTGI) [24] at 6- (T1), 12- (T2), and 24-month (T3) follow-up. Twenty-one items were assessed on a 6-point ordinal scale ranging from 0 (no change at all) to 5 (very great change) for 5 subscales: new possibilities (5 items), relating to others (7 items), personal strength (4 items), spiritual change (2 items), and appreciation of life (3 items). Cancer diagnosis was stated in the question stem as the reference for endorsing items. Scores for each of the five subscales were derived by summing response values, and all items were totaled to determine an overall summated PTGI score, ranging from 0 to 105. Higher scores indicated greater growth. We also computed the proportions of participants endorsing single items at a moderate or great level (item score ≥ 3) to be able to characterize the most common and frequent forms of PTG [25] and used a cut-off of ≥63 to classify low vs. high PTG levels as was done in previous studies [26–28]. Cronbach’s alpha ranged from .84 to .91 for the 5 subscales of the PTGI. Cronbach’s α was 0.95 for the overall score.
Social support was measured at baseline (T0) with the Medical Outcomes Survey Social Support survey, which is a 19-item questionnaire assessing the degree to which a chronically ill patient perceives availability of functional social support [29].
Posttraumatic stress symptoms were assessed at 6-, 12, and 24-month follow-up using the Posttraumatic Stress Diagnostic Scale (PDS) [30]. This measure includes 17 questions covering three categories of symptoms: re-experiencing (5 items), avoidance (7 items), and arousal (5 items). We adapted the items in the PDS by replacing the word “trauma” with “cancer” in order to specifically investigate cancer-related PTSS. Item scores are summed to derive an overall posttraumatic stress severity score ranging from 0 to 51.
Health-related quality of life (HRQoL) was measured at 24-month follow-up (T3) by the Medical Outcomes Study Short Form-36 Health Survey (SF-36) [31]. Two higher order component scores were calculated, one for physical health (Physical Component Score (PCS)) and one for mental health (Mental Component Score (MCS)). Based on 1998 US population data, raw scores of MCS and PCS are transformed into T scores ranging from 0 to 100, with higher scores representing better HRQoL.
Psychological distress was assessed at 24-month (T3) follow-up with the Brief Symptom Inventory-18 (BSI-18), which contains 18 self-report items [32] and comprises a Global Symptom Index (GSI). Raw scores for the GSI were converted to age- and sex-adjusted T scores for comparison to non-patient community norms (mean, 50; standard deviation, 10). Higher scores indicate greater distress.
Sociodemographic information, including age, sex, race, employment/school status, and relationship/marital status, was reported by patients. Clinical data obtained from medical charts included type of cancer, types of treatment (chemotherapy, radiation, and surgery), and treatment status (on versus off treatment). Surveillance, Epidemiology, and End Results (SEER) codes were used to categorize cancer type into severity of disease [33]. In addition, via self-report at baseline and 24-month follow-up, a symptom checklist, derived by the investigators, elicited subject endorsement of 11 common treatment-related side effects during the past month (i.e., shortness of breath; problems with memory, attention, or concentration; frequent or severe stomach pain, pain in your chest (heartburn), or indigestion; ringing in the ears; pain in your joints; weight loss; frequent fevers; lack of sleep or trouble sleeping; frequent tiredness or fatigue; frequent mouth sores that impact eating and drinking; frequent headaches), which were summed and considered in statistical analyses.
Statistical analyses
All statistical analyses were performed using SPSS software, version 22.0 (SPSS Inc., Chicago IL), and two-sided p values of <0.05 were considered statistically significant.
Differences in sociodemographic and clinical characteristics between patients who completed at least two follow-up questionnaires and those who did not were compared with chi-square or ANOVA, where appropriate. Patients who did not complete two follow-up questionnaires were excluded from further analyses.
Descriptive statistics were used to describe the mean PTG overall and subscale scores. Mean differences over time in PTG total and subscale scores were analyzed with paired-sample t tests. Thereafter, each respondent was assigned to a PTG trajectory group on the basis of PTGI total scores at each of three time points (6-, 12, and 24-month follow-up). AYAs whose PTG scores were ≥63 [26–28] at all three time points were assigned to the high PTG group. Those whose scores exceeded the threshold only at 24-month follow-up, or at 12- and 24-month follow-up, were assigned to the increasing PTG group. Those whose scores exceeded threshold only at 6-month follow-up, or at 6- and 12-month follow-up, were assigned to the decreasing PTG group. AYAs whose scores never reached the threshold were assigned to the low PTG group. Six patients had missing values at 12- or 24-month follow-up and were excluded from analysis.
To examine differences in sociodemographic, clinical, and psychosocial characteristics between the four groups, chi-square or ANOVA was used, where appropriate.
To determine the predictive and buffering value of PTG, three multiple hierarchical linear regression equations were estimated, one for each outcome (mental HRQoL, physical HRQoL, psychological distress), in four separate steps: (1) the control variables previously correlated at a significant level (p < 0.10) with the outcome measures, (2) adding PTS (mean centered), (3) adding PTG (mean centered), and (4) adding the interaction term PTS × PTG.
Results
Characteristics of the study population
The demographic and clinical characteristics of 169 participants who completed at least two follow-up surveys are summarized in Table 1. Of the 46 non-respondents, 28 were deceased (60.9%). Respondents were younger, more often received chemotherapy, and were on treatment at baseline compared to non-respondents and deceased patients. Non-respondents had a better overall survival expectation (based on 5-year survival data for each specific cancer type) compared to respondents.
Posttraumatic growth
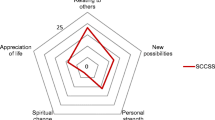
The average PTG total and subscale scores at each follow-up point are shown in Fig. 1. At T1, 59% scored high on PTG, while 64% at T2 and 58% at T3 scored ≥63. At 6-month follow-up, all AYAs reported at least one form of PTG, while at 12- and 24-month follow-up all but one AYA reported some form of PTG. The most frequently endorsed items were “An appreciation for the value of my own life” (83–87%), “Knowing I can handle difficulties” (77–81%), “Knowing that I can count on people in times of trouble” (72–86%), “Appreciate each day” (78–80%), “I discovered that I'm stronger than I thought I was” (69–81%), “I learned a great deal about how wonderful people are” (70–80%), and “My priorities about what is important in life” (71–73%). Paired-sample t tests showed no significant changes in PTG total scores or subscales over time but identified two subscales showing significant change: spiritual change (e.g., “I have a stronger religious faith”) which increased from 6 to 12 months (p = 0.006) and personal strength (e.g., “I discovered that I am stronger than I thought I was”) which improved from 6 to 12 months (p = 0.001) and 6 to 24 months (p = 0.003).
Among participants, 73 (45%) had high levels of PTG at all three time points (84, 83, and 86%, respectively), 23 (14%) had decreasing PTG levels (70, 64, and 42%, respectively), 23 (14%) had increasing PTG levels (48, 67, and 80%, respectively), and 44 (27%) had low PTG levels at all time points (38, 42, and 34%, respectively; Fig. 2).
Correlates
Participants who had high scores on PTG at each time point were more often female, younger, and receiving chemotherapy compared to the other three groups (low PTG, increasing PTG, decreasing PTG; Table 2). No differences between the groups were found in other sociodemographic and cancer-related factors.
Association between PTG and HRQoL and psychological distress
Table 3 summarizes the results of the hierarchical regression equations performed. Results show that PTG level at 6-month follow-up is predictive of mental HRQoL (β = 0.19; p = 0.026) and psychological distress (β = −0.14; p = 0.043) at 24-month follow-up when corrected for PTS at 6-month follow-up and covariates which were related to the outcomes on bivariate level. No relationship between PTG and physical HRQoL was found. Note that PTS also remained a significant predictor of mental HRQoL and psychological distress (Table 3).
Buffering effect of PTG on link between PTS and HRQoL and psychological distress
Results revealed no significant interaction effects between PTS and PTG on outcomes, indicating that buffering did not take place (Table 3).
Discussion
In this prospective follow-up study, we found that most AYAs with cancer experience some degree of PTG between 6 and 24 months after baseline assessment. In accordance with the literature, the most frequently scored PTGI items included a sense of enhanced life appreciation, reinforced interpersonal relationships, and personal strengthening, whereas finding new opportunities and spiritual change were the least endorsed areas of growth [25–27, 34, 35]. Our study adds to the current literature by showing that PTG is a dynamic process within individuals. Although no significant mean differences in PTG scores were found over time within the sample as a whole, we were able to identify four trajectories of individual change or stableness: high PTG, increasing PTG, decreasing PTG, and low PTG. Interestingly, the number of individuals who showed increasing PTG over time approximately matched those who showed decreasing PTG. A major goal for future work should be increasing the prevalence of increasing PTG profiles.
When looking at factors promoting or preventing PTG in different studies, our findings show that females, younger AYAs, and those who received chemotherapy more often were found in the high PTG group and least often in the low PTG group. Some researchers have suggested that women are, on average, more emotion-focused in their coping strategies and may therefore be more often able than men to derive a sense of positive meaning from the traumatic experience of cancer [10, 36]. Younger patients might be more aware of and motivated to conform to expectations to adopt a positive attitude in the face of cancer than are older patients [27, 37]. It may also be that a cancer diagnosis is more stressful and necessitates more developmental readjustment for younger AYAs [10], leading to more PTG. The other way around, the impact of cancer on younger AYAs is happening at a time when most of them still have support of their family/parents, they are just starting out on life, have not yet gained (financial) independence or are still reliant on parents, and do not have children or families of their own to worry about whilst those who are diagnosed as older AYAs may have all these responsibilities of having a job, family, and mortgage, which may potentially make a cancer diagnosis more life disrupting and may limit them to find benefit of their cancer experience. It could also be that older AYAs are more aware of what a cancer diagnosis may mean for them in terms of their health and their future. One might also hypothesize that receipt of more aversive treatments that produce greater life disruption (e.g., chemotherapy) might set the stage for greater PTG [10]; however, studies have demonstrated no association between chemotherapy and PTG [20, 26, 35].
Findings to date regarding PTG following a cancer diagnosis have been quite inconsistent regarding relationships with well-being; PTG is sometimes shown to be positively related to adjustment to cancer, but sometimes inversely, and often with little relationship at all [10, 19]. We found a direct positive predictive effect of PTG on mental HRQoL and a direct negative predictive effect on psychological distress. This suggests that PTG may reflect a cognitive adaptation process in response to a cancer diagnosis [19, 20], helping the AYAs to better mentally adjust to the cancer experience. PTG may provide a positive engagement with life, strengthening peoples’ feelings of self-reliance and fostering social relationships [38], which may explain these direct relationships. On the other hand, PTG was not a predictor of physical HRQoL. It seems that this outcome is more influenced by the physical symptoms AYAs are currently coping with than by PTG. One explanation for the lack of this relationship could be that PTGI measures psychosocial areas of growth and not physical domains [39]. Future research could explore the predictive value of physical HRQoL for PTG.
Inconsistent with previous research identifying PTG as a buffer against the harmful effects of PTS [34, 40], PTG did not interact with PTS to predict these outcomes in our study. It could be hypothesized that PTS and PTG consist of different processes which may co-exist but are largely independent [13, 26, 27, 41]. PTG reflects a coping process or resource that triggers the cognitive reconstruction of the individual’s beliefs and strengths, while PTS is more an emotional process of intrusive thoughts, avoidance behavior, and heightened arousability with an aversive nature [42]. However, there is one study showing that re-experiencing trauma (a symptom of PTS) has a curvilinear relationship with two scales of the PTGI (new possibilities and personal strengths), showing that re-experiencing may also represent a cognitive process necessary to achieve personal growth for AYAs [13]. The authors state that opportunities to engage family, friends, cancer survivors, or health care professionals in frank discussions about fears, worries, or concerns may help AYAs re-experience cancer in a way that enhances their understanding of what happened to them and contributes to better mental well-being.
One potential implication of these findings is that helping AYAs with cancer who experience low or decreasing levels of PTG find meaning and benefits in the cancer experience may help improve their mental adjustment. This may be accomplished by encouraging the reappraisal of the situation (positive reframing) or the emotional disclosure of inner feelings and fears [43]. A written emotional disclosure intervention, whereby adult breast cancer survivors were randomized to write about positive thoughts and feelings related to their cancer, showed that those who wrote down positive feelings reported less physician visits for medical comorbidities and distress than survivors who wrote down facts of their experience [43]. In addition, two cognitive-behavioral interventions among adult cancer patients, that included elements of coping skills training, relaxation exercises, conflict resolution, and emotional expression, had beneficial effects on PTG [44, 45]. Another way to enhance PTG may be regular physical activity. The physiological benefit of physical activity may be stress reduction. Physical activity may also increase levels of social support for AYA cancer patients and serve as an active coping strategy to decrease levels of distress [15]. In addition, physical activity can improve AYA cancer patients’ self-efficacy levels, whereby the experience of positive feelings may be enhanced. As PTG predicts mental well-being, interventions focused on improving PTG are likely beneficial in the early post-treatment period.
This study is limited by the fact that PTG trajectory group sizes were too small to conduct multivariate linear regression on outcomes. Therefore, we used mean-centered continuous PTG scores at 6-month follow-up to predict outcomes at 24-month follow-up. Longitudinal mixed model analyses were not conducted because of the lack of HRQoL data at 6-month follow-up. Furthermore, with regard to instrumentation, the current study relied upon generic instruments with limited evidence of validity and reliability in study samples consisting of persons under 18 years of age. In addition, we did not assess social desirability. Some cancer patients believe they should derive growth from their cancer experience and subsequently may report such growth regardless of their personal experience of positive change or benefit [41]. Similarly, some cancer patients know thinking and talking positively about their cancer experience will elicit positive attention from others [46]. Future research is needed to examine the meaning of PTG for AYAs in the broader contexts of their lives and the ways in which they process their experiences. For example, the four PTG trajectories could indicate that PTG functions as a coping style early after diagnosis but becomes real growth for some after a longer period of time [47].
In conclusion, this study shows that PTG is a dynamic process within the individual AYA cancer patient that predicts mental well-being outcomes but does not serve to buffer PTS. Psychosocial interventions should focus on both PTS and PTG in order to promote positive adjustment for AYAs diagnosed with cancer.
References
Adolescent and Young Adult Oncology Progress Review Group 2006. Closing the gap: research and care imperatives for adolescents and young adults with cancer. Bethesda, MD: National Institute of Health, National Cancer Institute, and Livestrong Young Ault Alliance, NIH Publication No. 06–6067
Haase JE, Phillips CR (2004) The adolescent/young adult experience. J Pediatr Oncol Nurs 21:145–149
Arnett JJ (2000) Emerging adulthood. A theory of development from the late teens through the twenties. Am Psychol 55:469–480
Thomas DM, Albritton KH, Ferrari A (2010) Adolescent and young adult oncology: an emerging field. J Clin Oncol 28:4781–4782
Zebrack BJ (2011) Psychological, social, and behavioral issues for young adults with cancer. Cancer 117:2289–2294
Smith AW, Bellizzi KM, Keegan TH et al (2013) Health-related quality of life of adolescent and young adult patients with cancer in the United States: the adolescent and young adult health outcomes and patient experience study. J Clin Oncol 31:2136–2145
Selye H (1956) The stress of life. McGraw-Hill, New York
Calhoun LG, Tedeschi RG (1999) Facilitating posttraumatic growth. A clinician’s guide. Lawrence Erlbaum Associates, Mahwah
Calhoun LG, Tedeschi RG (2002) Posttraumatic growth: the positive lessons of loss. In: Neimeyer RA (ed) Meaning reconstruction and the experience of loss. American Psychological Association, Washington, DC, pp 157–172
Stanton AL, Bower JE, Low CA (2006) Posttraumatic growth after cancer. In: Calhoun LG, Tedeschi RG (eds) Handbook of posttraumatic growth: research and practice. Erlbaum Associates, Mahwah, New Jersey, pp 138–175
Wicks L, Mitchell A (2010) The adolescent cancer experience: loss of control and benefit finding. Eur J Cancer Care (Engl) 19:778–785
Salsman JM, Garcia SF, Yanez B et al (2014) Physical, emotional, and social health differences between posttreatment young adults with cancer and matched healthy controls. Cancer 120:2247–2254
Zebrack B, Kwak M, Salsman J et al (2015) The relationship between posttraumatic stress and posttraumatic growth among adolescent and young adult (AYA) cancer patients. Psychooncology 24:162–168
Monteiro S, Torres A, Morgadinho R, Pereira A (2013) Psychosocial outcomes in young adults with cancer: emotional distress, quality of life and personal growth. Arch Psychiatr Nurs 27:299–305
Love C, Sabiston CM (2011) Exploring the links between physical activity and posttraumatic growth in young adult cancer survivors. Psychooncology 20:278–286
Seitz DC, Hagmann D, Besier T et al (2011) Life satisfaction in adult survivors of cancer during adolescence: what contributes to the latter satisfaction with life? Qual Life Res 20:225–236
Calhoun LG, Tedeschi RG (1995) Trauma and transformation: growing in the aftermath of suffering. SAGE Publications, Thousand Oaks, CA
Davis CG, Nolen-Hoeksema S, Larson J (1998) Making sense of loss and benefiting from the experience: two construals of meaning. J Pers Soc Psychol 75:561–574
Helgeson VS, Reynolds KA, Tomich PL (2006) A meta-analytic review of benefit finding and growth. J Consult Clin Psychol 74:797–816
Morrill EF, Brewer NT, O'Neill SC et al (2008) The interaction of post-traumatic growth and post-traumatic stress symptoms in predicting depressive symptoms and quality of life. Psychooncology 17:948–953
McCarthy MC, McNeil R, Drew S et al (2016) Psychological distress and posttraumatic stress symptoms in adolescents and young adults with cancer and their parents. J Adolesc Young Adult Oncol In press
Kwak M, Zebrack BJ, Meeske KA et al (2013) Prevalence and predictors of post-traumatic stress symptoms in adolescent and young adult cancer survivors: a 1-year follow-up study. Psychooncology 22:1798–1806
Meeske KA, Ruccione K, Globe DR, Stuber ML (2001) Posttraumatic stress, quality of life, and psychological distress in young adult survivors of childhood cancer. Oncol Nurs Forum 28:481–489
Tedeschi RG, Calhoun LG (1996) The posttraumatic growth inventory: measuring the positive legacy of trauma. J Trauma Stress 9:455–471
Gianinazzi ME, Rueegg CS, Vetsch J et al (2016) Cancer’s positive flip side: posttraumatic growth after childhood cancer. Support Care Cancer 24:195–203
Cordova MJ, Giese-Davis J, Golant M, Kronenwetter C, Chang V, Spiegel D (2007) Breast cancer as trauma: posttraumatic stress and posttraumatic growth. J Clin Psychol Med Settings 14:308–319
Widows MR, Jacobsen PB, Booth-Jones M, Fields KK (2005) Predictors of posttraumatic growth following bone marrow transplantation for cancer. Health Psychol 24:266–273
Jansen L, Hoffmeister M, Chang-Claude J et al (2011) Benefit finding and post-traumatic growth in long-term colorectal cancer survivors: prevalence, determinants, and associations with quality of life. Br J Cancer 105:1158–1165
Sherbourne CD, Stewart AL (1991) The MOS social support survey. Soc Sci Med 32:705–714
Foa EB, Cashman L, Jaycox L, Perry K (1997) The validation of a self-report measure of posttraumatic stress disorder: the posttraumatic diagnostic scale. Psychol Assess 9:445–451
Ware JE, Snow KK, Kosinski M et al (1993) SF-36 health survey: manual and interpretation guide. Boston, MA, The Health Institute, New England Medical Center
Derogatis LR (2001) Brief symptom inventory 18: administration, scoring, and procedures manual. Pearson, Bloomington
Howlader N, Noone AM, Krapcho M et al (2011) SEER cancer statistics review, 1975–2008. Bethesda, National Institutes of Health
Silva SM, Moreira HC, Canavarro MC (2012) Examining the links between perceived impact of breast cancer and psychosocial adjustment: the buffering role of posttraumatic growth. Psychooncology 21:409–418
Lelorain S, Bonnaud-Antignac A, Florin A (2010) Long term posttraumatic growth after breast cancer: prevalence, predictors and relationships with psychological health. J Clin Psychol Med Settings 17:14–22
Fife BL (1994) The conceptualization of meaning in illness. Soc Sci Med 38:309–316
Manne S, Ostroff J, Winkel G et al (2004) Posttraumatic growth after breast cancer: patient, partner, and couple perspectives. Psychosom Med 66:442–454
Pakenham KI (2005) Benefit finding in multiple sclerosis and associations with positive and negative outcomes. Health Psychol 24:123–132
Bellizzi KM, Smith AW, Reeve BB et al (2010) Posttraumatic growth and health-related quality of life in a racially diverse cohort of breast cancer survivors. J Health Psychol 15:615–626
Park CL, Chmielewski J, Blank TO (2010) Post-traumatic growth: finding positive meaning in cancer survivorship moderates the impact of intrusive thoughts on adjustment in younger adults. Psychooncology 19:1139–1147
Salsman JM, Segerstrom SC, Brechting EH et al (2009) Posttraumatic growth and PTSD symptomatology among colorectal cancer survivors: a 3-month longitudinal examination of cognitive processing. Psychooncology 18:30–41
Andrykowski MA, Cordova MJ, Studts JL, Miller TW (1998) Posttraumatic stress disorder after treatment for breast cancer: prevalence of diagnosis and use of the PTSD Checklist-Civilian Version (PCL-C) as a screening instrument. J Consult Clin Psychol 66:586–590
Stanton AL, Danoff-Burg S, Sworowski LA et al (2002) Randomized, controlled trial of written emotional expression and benefit finding in breast cancer patients. J Clin Oncol 20:4160–4168
Antoni MH, Lehman JM, Kilbourn KM et al (2001) Cognitive-behavioral stress management intervention decreases the prevalence of depression and enhances benefit finding among women under treatment for early-stage breast cancer. Health Psychol 20:20–32
Penedo FJ, Dahn JR, Molton I et al (2004) Cognitive-behavioral stress management improves stress-management skills and quality of life in men recovering from treatment of prostate carcinoma. Cancer 100:192–200
Wilkinson S, Kitzinger C (2000) Thinking differently about thinking positive: a discursive approach to cancer patients' talk. Soc Sci Med 50:797–811
Sawyer A, Ayers S, Field AP (2010) Posttraumatic growth and adjustment among individuals with cancer or HIV/AIDS: a meta-analysis. Clin Psychol Rev 30:436–447
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by HopeLab Foundation, Redwood City, CA. Dr. Olga Husson (KUN2015-7527) is supported by a Social Psychology Fellowship from the Dutch Cancer Society. None of these funders had control over study design, data collection, data analysis, and reporting. The authors have full control of all primary data and will allow the journal to review the data if requested.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Husson, O., Zebrack, B., Block, R. et al. Posttraumatic growth and well-being among adolescents and young adults (AYAs) with cancer: a longitudinal study. Support Care Cancer 25, 2881–2890 (2017). https://doi.org/10.1007/s00520-017-3707-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-017-3707-7