Abstract
Purpose
Patient-reported outcomes (PROs) can promote patient-centered care, but previous research has documented interpretation challenges among clinicians and patients. We engaged stakeholders to improve formats for presenting individual-level PRO data (for patient monitoring) and group-level PRO data (for reporting comparative clinical studies).
Methods
In an iterative process, investigators partnered with stakeholder workgroups of clinicians and patients to address previously identified interpretation challenges. Candidate approaches were then tested in semi-structured, one-on-one interviews with cancer patients and clinicians. Interpretation issues addressed included conveying score meaning (i.e., what is good/bad) and directional inconsistency (whether higher scores are better/worse). An additional issue for individual-level PROs was highlighting potentially concerning scores and, for group-level PROs, identifying important between-group differences (clinical, statistical).
Results
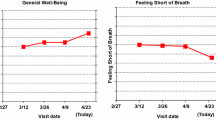
One-on-one interviews in a purposive sample of clinicians (n = 40) and patients (n = 39) provided insights regarding approaches to address issues identified. For example, adding descriptive labels to the Y-axis (none, mild, moderate, severe) helps address directional inconsistency and aids interpretation of score meaning. Red circles around concerning data points or a threshold line indicating worse-than-normal scores indicate possibly concerning scores for individual-level PRO data. For group-level PRO data, patients and some clinicians are confused by confidence limits and clinical versus statistical significance, but almost all clinicians want p values displayed.
Conclusions
Variations in interpretation accuracy demonstrate the importance of presenting PRO data in ways that promote understanding and use. In an iterative stakeholder-driven process, we developed improved PRO data presentation formats, which will be evaluated in further research across a large population of patients and clinicians.



Similar content being viewed by others
References
U.S. Food and Drug Administration (2009) Guidance for industry. Patient reported outcome measures: use in medical product development to support labeling claims. Fed Regist 74:65132–65133
Acquadro C, Berzon R, Dubois D et al (2001) Incorporating the patient’s perspective into drug development and communication: an ad hoc task force report of the Patient-Reported Outcomes (PRO) Harmonization Group meeting at the Food and Drug Administration, February 16, 2001. Value Health 6:522–531
Brundage MD, Feldman-Stewart D, Bezjak A et al (2011) The value of quality of life information in a cancer treatment decision. ISOQOL 11th annual conference, San Francisco, 2005
Brundage M, Bass B, Jolie R et al (2011) A knowledge translation challenge: clinical use of quality of life data from cancer clinical trials. Qual Life Res 20:979–985. doi:10.1007/s11136-011-9848-0
Snyder CF, Aaronson NK (2009) Use of patient-reported outcomes in clinical practice. Lancet 374:369–370. doi:10.1016/S0140-6736(09)61400-8
Greenhalgh J (2009) The applications of PROs in clinical practice: what are they, do they work, and why? Qual Life Res 18:115–123. doi:10.1007/s11136-008-9430-6
Velikova G, Booth L, Smith AB et al (2004) Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol 22:714–724. doi:10.1200/JCO.2004.06.078
Detmar SB, Muller MJ, Schornagel JH et al (2002) Health related quality of life assessments and patient physician communication. J Am Med Assoc 288:3027–3034. doi:10.1001/jama.288.23.3027
Snyder CF, Jensen R, Courtin SO et al (2009) PatientViewpoint: a website for patient-reported outcomes assessment. Qual Life Res 18:793–800. doi:10.1007/s11136-009-9497-8
Jones JB, Snyder CF, Wu AW (2007) Issues in the design of Internet-based systems for collecting patient-reported outcomes. Qual Life Res 16:1407–1417. doi:10.1007/s11136-007-9235-z
Snyder CF, Blackford AL, Wolff AC et al (2013) Feasibility and value of PatientViewpoint: a web system for patient-reported outcomes assessment in clinical practice. Psycho-Oncology 22:895–901. doi:10.1002/pon.3087
Brundage M, Smith KC, Little EA, Bantug ET, Snyder CF, PRO Data Presentation Stakeholder Advisory Board (2015) Communicating patient-reported outcome scores using graphic formats: results from a mixed-methods evaluation. Qual Life Res Online First. doi:10.1007/s11136-015-0974-y
PROMIS: Dynamic tools to measure health outcomes from the patient perspective. Available at nihpromis.org. Accessed 21 Aug 2015
Aaronson NK, Ahmedzai S, Bergman B et al (1993) The European organization for research and treatment of cancer QLQC30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
AtlasTi V7. Available at http://atlasti.com. Accessed 5 May 2016
Acknowledgments
This analysis was supported by a Patient-Centered Outcomes Research Institute (PCORI) Award (R-1410-24904). All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the PCORI and its Board of Governors or Methodology Committee. Dr. Smith and Dr. Snyder are members of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (P30 CA 006973). The funding sources had no role in the study design, data collection, analysis, interpretation, writing, or decision to submit the manuscript for publication. The PRO Data Presentation Stakeholder Advisory Board includes Neil K. Aaronson, PhD (Netherlands Cancer Institute); Patricia A. Ganz, MD (University of California-Los Angeles and Jonsson Comprehensive Cancer Center); Ravin Garg, MD (Anne Arundel Medical Center); Michael Fisch, MD (M.D. Anderson Cancer Center); Vanessa Hoffman, MPH (Bladder Cancer Advocacy Network); Bryce B. Reeve, PhD (University of North Carolina at Chapel Hill and Lineberger Comprehensive Cancer Center); Eden Stotsky-Himelfarb (Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins); Ellen Stovall (National Coalition for Cancer Survivorship); and Matthew Zachary (Stupid Cancer). The Johns Hopkins Clinical Research Network (JHCRN) site investigators and staff include Ravin Garg, MD, and Steven P. DeMartino, CCRC, CRT, RPFT (Anne Arundel Medical Center); Melissa Gerstenhaber, MAS, MSN, RN, CCRN (JHCRN/Anne Arundel Medical Center); Gary Cohen, MD, and Cynthia MacInnis, BS, CCRP (Greater Baltimore Medical Center); James Zabora, ScD, MSW (Inova Health System), and Sandra Schaefer, BSN, RN, OCN (JHCRN/Inova Health System); Paul Zorsky, MD, Lynne Armiger, MSN, CRNP, ANP-C, Sandra L. Heineken, BS, RN, OCN, and Nancy J. Mayonado, MS (Peninsula Regional Medical Center); Michael Carducci, MD (Johns Hopkins Sibley Memorial Hospital); and Carolyn Hendricks, MD, Melissa Hyman, RN, BSN, OCN, and Barbara Squiller, MSN, MPH, CRNP (Suburban Hospital). Finally, we are most grateful to the patients and clinicians who participated in this study. In particular, we extend our gratitude to the patients from part I of the study who agreed to contribute to the workgroups as research partners (rather than participants) for this phase of the research.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
The Johns Hopkins School of Medicine Institutional Review Board approved the study. All participants provided signed consent.
Rights and permissions
About this article
Cite this article
Smith, K.C., Brundage, M.D., Tolbert, E. et al. Engaging stakeholders to improve presentation of patient-reported outcomes data in clinical practice. Support Care Cancer 24, 4149–4157 (2016). https://doi.org/10.1007/s00520-016-3240-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3240-0