Abstract
Purpose
Tools to collect patient-reported outcome measures (PROMs) are frequently used in the healthcare setting to collect information that is most meaningful to patients. Due to discordance among how patients and healthcare providers rank symptoms that are considered most meaningful to the patient, engagement of patients in the development of PROMs is extremely important. This review aimed to identify studies that described how patients are involved in the item generation stage of cancer-specific PROM tools developed for cancer patients.
Methods
A literature search was conducted using keywords relevant to PROMs, cancer, and patient engagement. A manual search of relevant reference lists was also conducted. Inclusion criteria stipulated that publications must describe patient engagement in the item generation stage of development of cancer-specific PROM tools. Results were excluded if they were duplicate findings or non-English.
Results
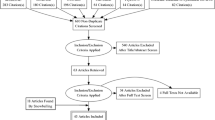
The initial search yielded 230 publications. After removal of duplicates and review of publications, 6 were deemed relevant. Fourteen additional publications were retrieved through a manual search of references from relevant publications. A total of 13 unique PROM tools that included patient input in item generation were identified. The most common method of patient engagement was through qualitative interviews or focus groups.
Conclusions
Despite recommendations from international groups and the emphasized importance of incorporating patient feedback in all stages of development of PROMs, few unique tools have incorporated patient input in item generation of cancer-specific tools. Moving forward, a framework of best practices on how to best engage patients in developing PROMs is warranted to support high-quality patient-centered care.

Similar content being viewed by others
References
U.S. Department of Health and Human Services FaDA, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, Center for Devices and Radiological Health (2009) Guidance for industry. use in medical product development to support labeling claims. Rockville, MD, Patient-reported outcome measures
Johnson C, Aaronson N, Blazeby JM, Bottomley A, Fayer P, Koller M, Kulis D, Ramage J, Sprangers M, Velikova G, Young T, Group obotEQoL (2011) EORTC Quality of Life Group—Guidelines for Developing Questionnaire Modules. 4th edn
Rothman M, Burke L, Erickson P, Leidy NK, Patrick DL, Petrie CD (2009) Use of existing patient-reported outcome (PRO) instruments and their modification: the ISPOR Good Research Practices for Evaluating and Documenting Content Validity for the Use of Existing Instruments and Their Modification PRO Task Force Report. Value Health 12(8):1075–1083. doi:10.1111/j.1524-4733.2009.00603.x
Cheville AL, Basford JR, Dos Santos K, Kroenke K (2014) Symptom burden and comorbidities impact the consistency of responses on patient-reported functional outcomes. Arch Phys Med Rehabil 95(1):79–86. doi:10.1016/j.apmr.2013.08.009
Macquart-Moulin G, Viens P, Bouscary ML, Genre D, Resbeut M, Gravis G, Camerlo J, Maraninchi D, Moatti JP (1997) Discordance between physicians’ estimations and breast cancer patients’ self-assessment of side-effects of chemotherapy: an issue for quality of care. Br J Cancer 76(12):1640–1645
Brunelli C, Costantini M, Di Giulio P, Gallucci M, Fusco F, Miccinesi G, Paci E, Peruselli C, Morino P, Piazza M, Tamburini M, Toscani F (1998) Quality-of-life evaluation: when do terminal cancer patients and health-care providers agree? J Pain Symptom Manag 15(3):151–158
Stephens RJ, Hopwood P, Girling DJ, Machin D (1997) Randomized trials with quality of life endpoints: are doctors’ ratings of patients’ physical symptoms interchangeable with patients’ self-ratings? Qual Life Res Int J Qual Life Asp Treat Care Rehab 6(3):225–236
Wagner LI, Robinson D Jr, Weiss M, Katz M, Greipp P, Fonseca R, Cella D (2012) Content development for the Functional Assessment of Cancer Therapy-Multiple Myeloma (FACT-MM): use of qualitative and quantitative methods for scale construction. J Pain Symptom Manag 43(6):1094–1104. doi:10.1016/j.jpainsymman.2011.06.019
Stromgren AS, Groenvold M, Pedersen L, Olsen AK, Spile M, Sjogren P (2001) Does the medical record cover the symptoms experienced by cancer patients receiving palliative care? A comparison of the record and patient self-rating. J Pain Symptom Manag 21(3):189–196
Stromgren AS, Groenvold M, Sorensen A, Andersen L (2001) Symptom recognition in advanced cancer. A comparison of nursing records against patient self-rating. Acta Anaesthesiol Scand 45(9):1080–1085
Garcia SF, Rosenbloom SK, Beaumont JL, Merkel D, Von Roenn JH, Rao D, Cella D (2012) Priority symptoms in advanced breast cancer: development and initial validation of the National Comprehensive Cancer Network-Functional Assessment of Cancer Therapy-Breast Cancer Symptom Index (NFBSI-16). Value Health 15(1):183–190. doi:10.1016/j.jval.2011.08.1739
Webster K, Cella D, Yost K (2003) The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Quality Life Outcomes 1:79. doi:10.1186/1477-7525-1-79
Lent L, Hahn E, Eremenco S, Webster K, Cella D (1999) Using cross-cultural input to adapt the Functional Assessment of Chronic Illness Therapy (FACIT) scales. Acta Oncol 38(6):695–702
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J, et al. (1993) The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol Off J Am Soc Clin Oncol 11(3):570–579
FACIT.org (2010) FACIT.org Questionnaires. FACIT.org. http://www.facit.org/FACITOrg/Questionnaires. Accessed November 9 2015
Flynn KE, Jeffery DD, Keefe FJ, Porter LS, Shelby RA, Fawzy MR, Gosselin TK, Reeve BB, Weinfurt KP (2011) Sexual functioning along the cancer continuum: focus group results from the Patient-Reported Outcomes Measurement Information System (PROMIS(R)). Psycho-Oncology 20(4):378–386. doi:10.1002/pon.1738
Flynn KE, Lin L, Cyranowski JM, Reeve BB, Reese JB, Jeffery DD, Smith AW, Porter LS, Dombeck CB, Bruner DW, Keefe FJ, Weinfurt KP (2013) Development of the NIH PROMIS Sexual Function and Satisfaction measures in patients with cancer. J Sex Med 10(Suppl 1):43–52. doi:10.1111/j.1743-6109.2012.02995.x
Chren MM, Lasek RJ, Quinn LM, Mostow EN, Zyzanski SJ (1996) Skindex, a quality-of-life measure for patients with skin disease: reliability, validity, and responsiveness. J Investigative Dermatol 107(5):707–713
Bates AS, Davis CR, Takwale A, Knepil GJ (2013) Patient-reported outcome measures in nonmelanoma skin cancer of the face: a systematic review. British J Dermatol 168(6):1187–1194. doi:10.1111/bjd.12269
Lee EH, Klassen AF, Nehal KS, Cano SJ, Waters J, Pusic AL (2013) A systematic review of patient-reported outcome instruments of nonmelanoma skin cancer in the dermatologic population. J Am Acad Dermatol 69(2):e59–e67. doi:10.1016/j.jaad.2012.09.017
Finlay AY, Khan GK (1994) Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol 19(3):210–216
Morgan M, McCreedy R, Simpson J, Hay RJ (1997) Dermatology quality of life scales—a measure of the impact of skin diseases. British J Dermatol 136(2):202–206
Matthews BA, Rhee JS, Neuburg M, Burzynski ML, Nattinger AB (2006) Development of the facial skin care index: a health-related outcomes index for skin cancer patients. Dermatol Surg 32(7):924–934 discussion 934. doi:10.1111/j.1524-4725.2006.32197.x
Rhee JS, Matthews BA, Neuburg M, Burzynski M, Nattinger AB (2005) Creation of a quality of life instrument for nonmelanoma skin cancer patients. Laryngoscope 115(7):1178–1185. doi:10.1097/01.mlg.0000166177.98414.5e
Rhee JS, Matthews BA, Neuburg M, Logan BR, Burzynski M, Nattinger AB (2006) Validation of a quality-of-life instrument for patients with nonmelanoma skin cancer. Arch Facial Plast Surg 8(5):314–318. doi:10.1001/archfaci.8.5.314
Rhee JS, Matthews BA, Neuburg M, Logan BR, Burzynski M, Nattinger AB (2007) The skin cancer index: clinical responsiveness and predictors of quality of life. Laryngoscope 117(3):399–405. doi:10.1097/MLG.0b013e31802e2d88
Burdon-Jones D, Thomas P, Baker R (2010) Quality of life issues in nonmetastatic skin cancer. British J Dermatol 162(1):147–151. doi:10.1111/j.1365-2133.2009.09469.x
Mathias SD, Chren MM, Colwell HH, Yim YM, Reyes C, Chen DM, Fosko SW (2014) Assessing health-related quality of life for advanced basal cell carcinoma and basal cell carcinoma nevus syndrome development of the first disease-specific patient-reported outcome questionnaires. JAMA Dermatol 150(2):169–176. doi:10.1001/jamadermatol.2013.5870
Cano SJ, Browne JP, Lamping DL, Roberts AH, McGrouther DA, Black NA (2006) The Patient Outcomes of Surgery-Head/Neck (POS-head/neck): a new patient-based outcome measure. J Plast Reconstr Aesthet Surg JPRAS 59(1):65–73
Kleinman L, Benjamin K, Viswanathan H, Mattera MS, Bosserman L, Blayney DW, Revicki DA (2012) The anemia impact measure (AIM): development and content validation of a patient-reported outcome measure of anemia symptoms and symptom impacts in cancer patients receiving chemotherapy. Qual Life Res Int J Qual Life Asp Treat Care Rehab 21(7):1255–1266. doi:10.1007/s11136-011-0034-1
Senn B, Gafner D, Happ MB, Eicher M, Mueller MD, Engberg S, Spirig R (2011) The unspoken disease: symptom experience in women with vulval neoplasia and surgical treatment: a qualitative study. European J Cancer Care 20(6):747–758. doi:10.1111/j.1365-2354.2011.01267.x
Senn B, Mueller MD, Hasenburg A, Blankenstein T, Kammermann B, Hartmann A, Donovan H, Eicher M, Spirig R, Engberg S (2012) Development of a postsurgical patient-reported outcome instrument for women with vulvar neoplasia. Oncol Nurs Forum 39(6):E489–E498. doi:10.1188/12.onf.e489-e498
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC, et al. (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85(5):365–376
Cancer EOfRaTo EORTC Quality of Life. EORTC Quality of Life Department. http://groups.eortc.be/qol/eortc-qlq-c30. Accessed November 9 2015
Portfolio CJ (2012) Navigation: a guide to implementing best practices in person-centred care. ON, Toronto
Acknowledgments
Production of this manuscript has been made possible through a financial contribution from Health Canada, through the Canadian Partnership Against Cancer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Camuso, N., Bajaj, P., Dudgeon, D. et al. Engaging Patients as Partners in Developing Patient-Reported Outcome Measures in Cancer—A Review of the Literature. Support Care Cancer 24, 3543–3549 (2016). https://doi.org/10.1007/s00520-016-3151-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3151-0