Abstract
Purpose
Cancer patients suffer from a variety of physical and mental complaints. Since physician assessment of symptoms seems insufficient to reveal the complete range of patients’ ailments, patient-reported outcomes (PRO) have become of key importance in modern cancer treatment. The implementation and first results of a systematic electronic real-time assessment of PRO in routine care is described.
Methods
Consecutive patients presenting for the first time to a German comprehensive cancer center were asked to fill in an adaptive self-administered electronic questionnaire consisting of standardized PRO measures. After completion, patient-reported data was linked to the patients’ medical files for discussion in the first consultation with the treating physician. Interviews with staff were conducted to identify barriers in implementation.
Results
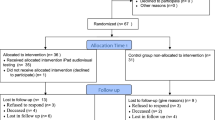
Out of 160 cancer patients, 126 (79 %; mean age 63 years, 67 % males) agreed to participate. The number of recruited patients increased over time. Of participating patients, 67 % provided complete information on all PRO-related scales. On average, 31 min (range 3–140) were required to fill in the questionnaire. Of participating patients, 53.0 % comprised need for psychooncological support and 62 % revealed moderate to severe psychosocial distress. The mean score for global quality of life according to the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30) was 55.2 (SD ±25.6).
Conclusions
Comprehensive oncological treatment needs to consider disease symptoms, quality of life, preferences, and comorbidities of individual patients in a structured, standardized, and transparent way. Our findings indicate that an adaptive, self-administered electronic assessment tool for cancer patients to report a broad set of PRO can be feasibly implemented and is well accepted by patients in a realistic setting.



Similar content being viewed by others
References
Smith AW, Reeve BB, Bellizzi KM, et al. (2008) Cancer, comorbidities, and health-related quality of life of older adults. Health Care Financ Rev 29:41–56
Hewitt M, Rowland JH, Yancik R (2003) Cancer survivors in the United States: age, health, and disability. J Gerontol A Biol Sci Med Sci 58:82–91
Singer S, Das-Munshi J, Brähler E (2010) Prevalence of mental health conditions in cancer patients in acute care—a meta-analysis. Ann Oncol off J Eur Soc Med Oncol ESMO 21:925–930. doi:10.1093/annonc/mdp515
Carlson LE, Angen M, Cullum J, et al. (2004) High levels of untreated distress and fatigue in cancer patients. Br J Cancer 90:2297–2304. doi:10.1038/sj.bjc.6601887
Fromme EK, Eilers KM, Mori M, et al. (2004) How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the quality-of-life questionnaire C30. J Clin Oncol off J Am Soc Clin Oncol 22:3485–3490. doi:10.1200/JCO.2004.03.025
Efficace F, Rosti G, Aaronson N, et al. (2014) Patient- versus physician-reporting of symptoms and health status in chronic myeloid leukemia. Haematologica 99:788–793. doi:10.3324/haematol.2013.093724
Di Maio M, Gallo C, Leighl NB, et al. (2015) Symptomatic toxicities experienced during anticancer treatment: agreement between patient and physician reporting in three randomized trials. J Clin Oncol Off J Am Soc Clin Oncol. doi:10.1200/JCO.2014.57.9334
Laugsand EA, Sprangers MA, Bjordal K, et al. (2010) Health care providers underestimate symptom intensities of cancer patients: a multicenter European study. Health Qual Life Outcomes 8:104. doi:10.1186/1477-7525-8-104
Quinten C, Maringwa J, Gotay CC, et al. (2011) Patient self-reports of symptoms and clinician ratings as predictors of overall cancer survival. J Natl Cancer Inst 103:1851–1858. doi:10.1093/jnci/djr485
Tariman JD, Berry DL, Cochrane B, et al. (2010) Preferred and actual participation roles during health care decision making in persons with cancer: a systematic review. Ann Oncol 21:1145–1151. doi:10.1093/annonc/mdp534
U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health (2006) Guidance for industry: patient-reported outcome measures: use in medical product development to supportlabeling claims: draft guidance. Health Qual Life Outcomes 4:79. doi:10.1186/1477-7525-4-79
Howell D, Molloy S, Wilkinson K, et al. (2015) Patient-reported outcomes in routine cancer clinical practice: a scoping review of use, impact on health outcomes, and implementation factors. Ann Oncol Off J Eur Soc Med Oncol ESMO. doi:10.1093/annonc/mdv181
Chen J, Ou L, Hollis SJ (2013) A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res 13:211. doi:10.1186/1472-6963-13-211
Luckett T, Butow PN, King MT (2009) Improving patient outcomes through the routine use of patient-reported data in cancer clinics: future directions. Psychooncology 18:1129–1138. doi:10.1002/pon.1545
Kotronoulas G, Kearney N, Maguire R, et al. (2014) What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol 32:1480–1501. doi:10.1200/JCO.2013.53.5948
Marshall S, Haywood K, Fitzpatrick R (2006) Impact of patient-reported outcome measures on routine practice: a structured review. J Eval Clin Pract 12:559–568. doi:10.1111/j.1365-2753.2006.00650.x
Kallen MA, Yang D, Haas N (2011) A technical solution to improving palliative and hospice care. Support Care Cancer 20:167–174. doi:10.1007/s00520-011-1086-z
Boyes A, Newell S, Girgis A, et al. (2006) Does routine assessment and real-time feedback improve cancer patients’ psychosocial well-being? Eur J Cancer Care (Engl) 15:163–171. doi:10.1111/j.1365-2354.2005.00633.x
Hilarius DL, Kloeg PH, Gundy CM, Aaronson NK (2008) Use of health-related quality-of-life assessments in daily clinical oncology nursing practice. Cancer 113:628–637. doi:10.1002/cncr.23623
Antunes B, Harding R, Higginson IJ, EUROIMPACT (2014) Implementing patient-reported outcome measures in palliative care clinical practice: a systematic review of facilitators and barriers. Palliat Med 28:158–175. doi:10.1177/0269216313491619
Bundesministerium für Gesundheit (2012) Nationaler Krebsplan: Handlungsfelder. Ziele und, Umsetzungsempfehlungen. Berlin
Federal Joint Committee (GBA) 2016 Spezialisierte Ambulante Palliativversorgungs-Richtlinie/SAPV-RL.
Bausewein C, Simon ST, Benalia H, et al. (2011) Implementing patient reported outcome measures (PROMs) in palliative care—users’ cry for help. Health Qual Life Outcomes 9:27. doi:10.1186/1477-7525-9-27
Daveson BA, Simon ST, Benalia H, et al. (2012) Are we heading in the same direction? European and African doctors’ and nurses’ views and experiences regarding outcome measurement in palliative care. Palliat Med 26:242–249. doi:10.1177/0269216311409614
Cleeland CS (2009) The Brief Pain Inventory User Guide. M.D. Anderson Cancer Center, Houston
Cleeland CS, Ryan KM (1994) Pain assessment: global use of the brief pain inventory. Ann Acad Med Singap 23:129–138
Aaronson NK, Ahmedzai S, Bergman B, et al. (1993) The European Organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Roth AJ, Kornblith AB, Batel-Copel L, et al. (1998) Rapid screening for psychologic distress in men with prostate carcinoma: a pilot study. Cancer 82:1904–1908
Mehnert A, Müller D, Lehmann C, Koch U (2006) Die deutsche version des NCCN distress-thermometers: empirische Prüfung eines screening-instruments zur erfassung psychosozialer belastung bei krebspatienten. Z Für Psychiatr Psychol Psychother 54:213–223. doi:10.1024/1661-4747.54.3.213
Strittmatter G, Mawick R, Tilkorn M (2000) Entwicklung und klinischer einsatz von screening-instrumenten zur identifikation betreuungsbedürftiger tumorpatienten. In: Leb. Aus Med. – Soziol. Perspekt. Hogrefe, Göttingen, Bern, Toronto, Seattle, pp 59–75
Rubenstein LZ, Harker JO, Salvà A, et al. (2001) Screening for undernutrition in geriatric practice developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci 56:M366–M372. doi:10.1093/gerona/56.6.M366
Karnofsky DA, Abelmann WH, Craver LF, Burchenal JH (1948) The use of the nitrogen mustards in the palliative treatment of carcinoma with particular reference to bronchogenic carcinoma cancer. 1:634–656. doi:10.1002/1097-0142(194811)1:4<634::AID-CNCR2820010410>3.0.CO;2-L
Verger E, Salamero M (1990) Conill C (1992) can karnofsky performance status be transformed to the Eastern Cooperative Oncology Group scoring scale and vice versa? Eur J Cancer Oxf Engl 28A:1328–1330
Degner LF, Sloan JA, Venkatesh P (1997) The control preferences scale. Can J Nurs Res Rev can Rech En Sci Infirm 29:21–43
Herschbach P, Weis J (2010) Screeningverfahren in der Psychoonkologie Testinstrumente zur Identifikation betreuungsbedürftiger Krebspatienten-Eine Empfehlung der PSO für die psychoonkologische Behandlungspraxis.
Kaiser MJ, Bauer JM, Ramsch C, et al. (2009) Validation of the mini nutritional assessment short-form (MNA®-SF): a practical tool for identification of nutritional status. JNHA - J Nutr Health Aging 13:782–788. doi:10.1007/s12603-009-0214-7
Cocks K, King MT, Velikova G, et al. (2012) Evidence-based guidelines for interpreting change scores for the European organisation for the research and treatment of cancer quality of life questionnaire Core 30. Eur J Cancer 48:1713–1721. doi:10.1016/j.ejca.2012.02.059
Holzner B, Giesinger JM, Pinggera J, et al. (2012) The computer-based Health evaluation software (CHES): a software for electronic patient-reported outcome monitoring. BMC Med Inform Decis Mak 12:126. doi:10.1186/1472-6947-12-126
Ashley L, Jones H, Forman D, et al. (2011) Feasibility test of a UK-scalable electronic system for regular collection of patient-reported outcome measures and linkage with clinical cancer registry data: the electronic patient-reported outcomes from cancer survivors (ePOCS) system. BMC Med Inform Decis Mak 11:66. doi:10.1186/1472-6947-11-66
Van de Poll-Franse LV, Horevoorts N, van Eenbergen M, et al. (1990) (2011) the patient reported outcomes following initial treatment and long term evaluation of survivorship registry: scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur J Cancer Oxf Engl 47:2188–2194. doi:10.1016/j.ejca.2011.04.034
De Bree R, Verdonck-de Leeuw IM, Al K, et al. (2008) Touch screen computer-assisted health-related quality of life and distress data collection in head and neck cancer patients. Clin Otolaryngol 33:138–142. doi:10.1111/j.1749-4486.2008.01676.x
Zagadailov E, Fine M, Shields A (2013) Patient-reported outcomes are changing the landscape in Oncology Care: challenges and opportunities for payers. Am Health Drug Benefits 6:264–274
Berry DL, Hong F, Halpenny B, et al. (2014) Electronic self-report assessment for Cancer And Self-Care support: results of a multicenter randomized trial. J Clin Oncol 32:199–205. doi:10.1200/JCO.2013.48.6662
Carlson LE, Groff SL, Maciejewski O, Bultz BD (2010) Screening for distress in lung and breast cancer outpatients: a randomized controlled trial. J Clin Oncol off J Am Soc Clin Oncol 28:4884–4891. doi:10.1200/JCO.2009.27.3698
Morris J, Perez D, McNoe B (1998) The use of quality of life data in clinical practice. Qual Life Res Int J Qual Life Asp Treat Care Rehabil 7:85–91
Mitchell AJ (2007) Pooled results from 38 analyses of the accuracy of distress thermometer and other ultra-short methods of detecting cancer-related mood disorders. J Clin Oncol 25:4670–4681. doi:10.1200/JCO.2006.10.0438
Hinz A, Singer S, Brähler E (2014) European reference values for the quality of life questionnaire EORTC QLQ-C30: results of a German investigation and a summarizing analysis of six European general population normative studies. Acta Oncol 1–8. doi:10.3109/0284186X.2013.879998
van den Everdingen M B, de Rijke J, Kessels AG, et al. (2007) Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 18:1437–1449. doi:10.1093/annonc/mdm056
Snyder CF, Jensen R, Courtin SO, Wu AW (2009) PatientViewpoint: a website for patient-reported outcomes assessment. Qual Life Res Int J Qual Life Asp Treat Care Rehabil 18:793–800. doi:10.1007/s11136-009-9497-8
Atreja A, Rizk M (2012) Capturing patient reported outcomes and quality of life in routine clinical practice: ready for prime time? Minerva Gastroenterol Dietol 58:19–24
Petersen MA, Groenvold M, Aaronson NK, et al. (2010) Development of computerised adaptive testing (CAT) for the EORTC QLQ-C30 dimensions—general approach and initial results for physical functioning. Eur J Cancer 46:1352–1358. doi:10.1016/j.ejca.2010.02.011
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest and are in full control of all primary data.
Additional information
Freya Trautmann, Leopold Hentschel, Jochen Schmitt, and Markus Schuler have contributed equally.
Rights and permissions
About this article
Cite this article
Trautmann, F., Hentschel, L., Hornemann, B. et al. Electronic real-time assessment of patient-reported outcomes in routine care—first findings and experiences from the implementation in a comprehensive cancer center. Support Care Cancer 24, 3047–3056 (2016). https://doi.org/10.1007/s00520-016-3127-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3127-0