Abstract
Purpose
Intravenous catheters are used for the administration of intravenous therapy and for blood sampling. These devices are considered as well-functioning if both the injection and aspiration are easy. Malfunction is frequently observed and usually vaguely described as occlusion. We developed the CINAS, the Catheter Injection and Aspiration scheme. The CINAS is a catheter function classification tool, which classifies both the injection and the aspiration ability in a uniform way. Each CINAS class consists of a combination of an injection (IN) and an aspiration (AS) code: e.g. IN1AS1 is the CINAS class for a well-functioning catheter. In this series, we aimed to determine the accuracy of the CINAS class reported by nurses, after minimal training, versus a trained researcher, acting as a reference standard.
Methods
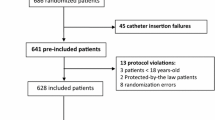
Catheter function was assessed during a standard blood sampling procedure through a totally implantable venous access device in a convenience sample of 150 oncology patients. One nurse researcher and 111 oncology nurses both scored the catheter function according to the CINAS classification scheme, independently. Concordance between the scores was calculated.
Results
For the 140 catheters scored as well-functioning (IN1AS1 score) by the researcher, 139 or 99.3 % (95 % confidence interval (CI) 96.1–99.9 %) were scored correctly by the nurse participants. Nine out of ten or 90 % (95 % CI 55.5–98.3 %) of malfunctioning catheters (researcher scores different from IN1AS1) were also identified as malfunctioning by the nurse participants and received exactly the same CINAS score in eight cases (80 %, 95 % CI 44.4–97.5 %). The overall accuracy of the CINAS scored by the nurse participants versus the researcher is (139 + 9)/150 or 98.7 % (95 % CI 95.3–99.8 %).
Conclusions
Nurse participants were able to classify the catheter function of totally implantable venous access devices with the CINAS accurately after a brief explanation about the classification options.

Similar content being viewed by others
References
Goossens GA, Stas M, Jerome M, Moons P (2011) Systematic review: malfunction of totally implantable venous access devices in cancer patients. Support Care Cancer 19:883–898. doi:10.1007/s00520-011-1171-3
Pikwer A, Baath L, Davidson B, Perstoft I, Akeson J (2008) The incidence and risk of central venous catheter malpositioning: a prospective cohort study in 1619 patients. Anaesth Intensive Care 36:30–37
Skaff ER, Doucette S, McDiarmid S, Huebsch L, Sabloff M (2012) Vascular access devices in leukemia: a retrospective review amongst patients treated at the Ottawa Hospital with induction chemotherapy for acute leukemia. Leuk Lymphoma 53:1090–1095. doi:10.3109/10428194.2011.639879
Cesaro S, Corro R, Pelosin A, Gamba P, Zadra N, Fusaro F, Pillon M, Cusinato R, Zampieri C, Magagna L, Cavaliere M, Tridello G, Zanon G, Zanesco L (2004) A prospective survey on incidence and outcome of Broviac/Hickman catheter-related complications in pediatric patients affected by hematological and oncological diseases. Ann Hematol 83:183–188. doi:10.1007/s00277-003-0796-9
Lim MY, Al-Kali A, Ashrani AA, Begna KH, Elliott MA, Hogan WJ, Hook CC, Kaufmann SH, Letendre L, Litzow MR, Patnaik MS, Pardanani A, Tefferi A, Wolanskyj AP, Grill DE, Pruthi RK (2013) Comparison of complication rates of Hickman (®) catheters versus peripherally inserted central catheters in patients with acute myeloid leukemia undergoing induction chemotherapy. Leuk Lymphoma 54:1263–1267. doi:10.3109/10428194.2012.742520
Surov A, Wienke A, Carter JM, Stoevesandt D, Behrmann C, Spielmann RP, Werdan K, Buerke M (2009) Intravascular embolization of venous catheter—causes, clinical signs, and management: a systematic review. JPEN J Parenter Enteral Nutr 33:677–685. doi:10.1177/0148607109335121
Stephens LC, Haire WD, Kotulak GD (1995) Are clinical signs accurate indicators of the cause of central venous catheter occlusion? JPEN J Parenter Enteral Nutr 19:75–79
Baskin JL, Pui CH, Reiss U, Wilimas JA, Metzger ML, Ribeiro RC, Howard SC (2009) Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet 374:159–169
Nakazawa N (2010) Infectious and thrombotic complications of central venous catheters. Semin Oncol Nurs 26:121–131
Goossens GA, Vrebos M, De Wever I, Stas M (2004) Vacutainer filling time through subcutaneous venous access devices. J Vasc Access 5:154–160
Verhamme P, Goossens G, Maleux G, Collen D, Stas M (2007) A dose-finding clinical trial of staphylokinase SY162 in patients with long-term venous access catheter thrombotic occlusion. J Thromb Thrombolysis 24:1–5
Horne MK III, Mayo DJ (1997) Low-dose urokinase infusions to treat fibrinous obstruction of venous access devices in cancer patients. J Clin Oncol 15:2709–2714
Frank JL, Garb JL, Halla B, Reed WP Jr (2001) Ionic implantation of silicone chronic venous access devices does not alter thrombotic complications: a double-blinded, randomized clinical trial. Surgery 129:547–551
Carlo JT, Lamont JP, McCarty TM, Livingston S, Kuhn JA (2004) A prospective randomized trial demonstrating valved implantable ports have fewer complications and lower overall cost than nonvalved implantable ports. Am J Surg 188:722–727
Stevens B, Barton SE, Brechbill M, Moenter S, Piel AL, Shankle D (2000) A randomized, prospective trial of conventional vascular ports vs. the vortex “clear-flow” reservoir port in adult oncology patients. JVAD 5(4):37–40
Johansson E, Bjorkholm M, Bjorvell H, Hast R, Takolander R, Olofsson P, Backman L, Weitzberg E, Engervall P (2004) Totally implantable subcutaneous port system versus central venous catheter placed before induction chemotherapy in patients with acute leukaemia—a randomized study. Support Care Cancer 12:99–105
Goossens GA, Stas M, Moons P (2012) Management of functional complications of totally implantable venous access devices by an advanced practice nursing team: 5 years of clinical experience. Eur J Oncol Nurs S1462-3889(11):00173–6. doi:10.1016/j.ejon.2011.11.006
Sauerland C, Engelking C, Wickham R, Corbi D (2006) Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum 33:1134–1141
Goossens GA, Kerschaever I, Despierre E, Stas M (2013) Access of a fully rotated implantable port leads to extravasation. J Vasc Access 14:299–300. doi:10.5301/jva.5000136, C4F9D654-8A58-4478-A3A5-603E3616D9DB
Infusion Nursing Standards of Practice (2011) J Infus Nurs 34:S1–110
Perez Fidalgo JA, Garcia FL, Cervantes A, Margulies A, Vidall C, Roila F (2012) Management of chemotherapy extravasation: ESMO–EONS Clinical Practice Guidelines. Eur J Oncol Nurs 16:528–534
Reitsma JB, Rutjes AW, Khan KS, Coomarasamy A, Bossuyt PM (2009) A review of solutions for diagnostic accuracy studies with an imperfect or missing reference standard. J Clin Epidemiol 62:797–806. doi:10.1016/j.jclinepi.2009.02.005, S0895-4356(09)00063-8
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goossens, G.A., De Waele, Y., Jérôme, M. et al. Diagnostic accuracy of the Catheter Injection and Aspiration (CINAS) classification for assessing the function of totally implantable venous access devices. Support Care Cancer 24, 755–761 (2016). https://doi.org/10.1007/s00520-015-2839-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2839-x