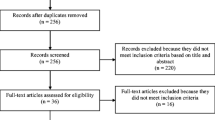
Abstract
Purpose
Various instruments are used to assess both individual and multiple cancer symptoms. We evaluated the psychometric properties of cancer multisymptom assessment instruments.
Methods
An Ovid MEDLINE search was done. All searches were limited to adults and in English. All instruments published from 2005 to 2014 (and with at least one validity test) were included. We excluded those who only reported content validity. Instruments were categorized by the three major types of symptom measurement scales employed as follows: visual analogue (VAS), verbal rating (VRS), and numerical rating (NRS) scales. They were then examined in two areas: (1) psychometric thoroughness (number of tests) and (2) psychometric strength of evidence (validity, reliability, generalizability). We also assigned an empirical global psychometric quality score (which combined the concepts of thoroughness and strength of evidence) to rank the instruments.
Results
We analyzed 57 instruments (17 original, 40 modifications). They varied in types of scales used, symptom dimensions measured, and time frames evaluated. Of the 57, 10 used VAS, 28 VRS, and 19 NRS. The Edmonton Symptom Assessment System (ESAS), ESAS-Spanish, Hospital Anxiety and Depression Scale (HADS), Profile of Mood States (POMS), Symptom Distress Scale (SDS), M.D. Anderson Symptom Inventory (MDASI)-Russian, and MDASI-Taiwanese were the most comprehensively tested for validity and reliability. The ESAS, ESAS-Spanish, ASDS-2, Memorial Symptom Assessment Scale (MSAS)-SF, POMS, SDS, MDASI (and some translations), and MDASI-Heart Failure all showed good validity and reliability.
Conclusions
The MDASI appeared to be the best overall from a psychometric perspective. This was followed by the ESAS, ESAS-Spanish, POMS, SDS, and some MDASI translations. VRS-based instruments were most common. There was a wide range of psychometric rigor in validation. Consequently, meta-analysis was not possible. Most cancer multisymptom assessment instruments need further extensive validation to establish the excellent reliability and validity required for clinical utility and meaningful research.


Similar content being viewed by others
References
(FDA) FaDA. Food and Drug Administration (FDA) (2011) Guidance for industry. Patient-reported outcome measures: use in medical product development to support labeling claims. In: Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf. hwfgd, editor. Accessed 24 May 20132011
Sloan JA, Berk L, Roscoe J, Fisch MJ, Shaw EG, Wyatt G et al (2007) Integrating patient-reported outcomes into cancer symptom management clinical trials supported by the National Cancer Institute-sponsored clinical trials networks. J Clin Oncol 25(32):5070–5077
Cleeland CS (2007) Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr 37:16–21
Greenhalgh J, Meadows K (1999) The effectiveness of the use of patient-based measures of health in routine practice in improving the process and outcomes of patient care: a literature review. J Eval Clin Pract 5(4):401–416
Policy CfMT. Center for Medical Technology Policy. Recommendations for incorporating patient-reported outcomes (PROs) into clinical comparative effectiveness research (CER) in adult oncology. 2013. http://www.cmtp.net.org/wp-content/uploads/downloads/2012/05/PRO-EGDpdf [Internet]. 2013
Frost MH, Reeve BB, Liepa AM, Stauffer JW, Hays RD (2007) What is sufficient evidence for the reliability and validity of patient-reported outcome measures? Value Health 10(Suppl 2):S94–S105
DeVon HA, Block ME, Moyle-Wright P, Ernst DM, Hayden SJ, Lazzara DJ et al (2007) A psychometric toolbox for testing validity and reliability. J Nurs Scholarsh 39(2):155–164
Swartz RJ, Schwartz C, Basch E, Cai L, Fairclough DL, McLeod L et al (2011) The king's foot of patient-reported outcomes: current practices and new developments for the measurement of change. Qual Life Res 20(8):1159–1167
Kirkova J, Davis MP, Walsh D, Tiernan E, O'Leary N, LeGrand SB et al (2006) Cancer symptom assessment instruments: a systematic review. J Clin Oncol 24(9):1459–1473
McDowel I (2006) Measuring health: a guide to rating scales and questionnaires. the theoretical and technical foundations of health measurement, 3rd edn. Oxford University Press, New York, pp 10–54
Lipscomb J, Snyder CF, Gotay CC (2007) Cancer outcomes measurement: through the lens of the Medical Outcomes Trust framework. Qual Life Res 16(1):143–164
Bowling A (2005) Theory of measurement. measuring health: a review of quality of life measurement scales, 3rd edn. Open University Press, Berkshire, pp 10–18
Streiner DL, GN (2003) Validity. Health measurement scales a practical guide to their development and use, New York, United States, Oxford University Press, pp. 172-93
Bourbonnais FF, Perreault A, Bouvette M (2004) Introduction of a pain and symptom assessment tool in the clinical setting–lessons learned. J Nurs Manag 12(3):194–200
de Vet HC, Ader HJ, Terwee CB, Pouwer F (2005) Are factor analytical techniques used appropriately in the validation of health status questionnaires? A systematic review on the quality of factor analysis of the SF-36. Qual Life Res 14(5):1203–1218, dicussion 19-21, 23-4
Mundfrom DSD, Ke TL (2005) Minimum sample size recommendations for conducting factor analysis. Int J Test 5:159–168
Le Pechoux C, Laplanche A, Faivre-Finn C, Ciuleanu T, Wanders R, Lerouge D et al (2011) Clinical neurological outcome and quality of life among patients with limited small-cell cancer treated with two different doses of prophylactic cranial irradiation in the intergroup phase III trial (PCI99-01, EORTC 22003-08004, RTOG 0212 and IFCT 99-01). Ann Oncol 22(5):1154–1163
Tucci RA, Bartels KL (1998) Patient use of the symptom reporting tool. Clin J Oncol Nurs 2(3):97–99
Kutner JS, Kassner CT, Nowels DE (2001) Symptom burden at the end of life: hospice providers' perceptions. J Pain Symptom Manage 21(6):473–480
Edmonds PM, Stuttaford JM, Penny J, Lynch AM, Chamberlain J (1998) Do hospital palliative care teams improve symptom control? Use of a modified STAS as an evaluation tool. Palliat Med 12(5):345–351
Kuebler KK, KO (1998) Psychometric evaluation of an objective assessment instrument to measure pain, dyspnea and restlessness. J Palliat Care Report from 12th International Congress on Care of the Terminally ill 14:125
McMillan SC, Moody LE (2003) Hospice patient and caregiver congruence in reporting patients' symptom intensity. Cancer Nurs 26(2):113–118
Given B, Given CW, McCorkle R, Kozachik S, Cimprich B, Rahbar MH et al (2002) Pain and fatigue management: results of a nursing randomized clinical trial. Oncol Nurs Forum 29(6):949–956
Nekolaichuk CL, Maguire TO, Suarez-Almazor M, Rogers WT, Bruera E (1999) Assessing the reliability of patient, nurse, and family caregiver symptom ratings in hospitalized advanced cancer patients. J Clin Oncol 17(11):3621–3630
Nekolaichuk CL, Bruera E, Spachynski K, MacEachern T, Hanson J, Maguire TO (1999) A comparison of patient and proxy symptom assessments in advanced cancer patients. Palliat Med 13(4):311–323
Ivanova MO, Ionova TI, Kalyadina SA, Uspenskaya OS, Kishtovich AV, Guo H et al (2005) Cancer-related symptom assessment in Russia: validation and utility of the Russian M. D. Anderson Symptom Inventory. J Pain Symptom Manage 30(5):443–453
Lin CC, Chang AP, Cleeland CS, Mendoza TR, Wang XS (2007) Taiwanese version of the M. D. Anderson symptom inventory: symptom assessment in cancer patients. J Pain Symptom Manage 33(2):180–188
Moro C, Brunelli C, Miccinesi G, Fallai M, Morino P, Piazza M et al (2006) Edmonton symptom assessment scale: Italian validation in two palliative care settings. Support Care Cancer 14(1):30–37
Rhodes VA, McDaniel RW (1999) The index of nausea, vomiting, and Retching: a new format of the lndex of Nausea and Vomiting. Oncol Nurs Forum 26(5):889–894
Wang XS, Laudico AV, Guo H, Mendoza TR, Matsuda ML, Yosuico VD et al (2006) Filipino version of the M. D. Anderson Symptom Inventory: validation and multisymptom measurement in cancer patients. J Pain Symptom Manage 31(6):542–552
Yun YH, Mendoza TR, Kang IO, You CH, Roh JW, Lee CG et al (2006) Validation study of the Korean version of the M. D. Anderson Symptom Inventory. J Pain Symptom Manage 31(4):345–352
Fu MR, Rhodes V, Xu B (2002) The Chinese translation of the index of nausea, vomiting, and retching. Cancer Nurs 25(2):134–140
Baba K, Fransson P, Lindh J (2007) Use of a modified ESAS in cancer patients: a pilot study of patient and staff experiences. Int J Palliat Nurs 13(12):610–616
Fadol A, Mendoza T, Gning I, Kernicki J, Symes L, Cleeland CS et al (2008) Psychometric testing of the MDASI-HF: a symptom assessment instrument for patients with cancer and concurrent heart failure. J Card Fail 14(6):497–507
Armstrong TS, Gning I, Mendoza TR, Weinberg JS, Gilbert MR, Tortorice ML et al (2009) Clinical utility of the MDASI-BT in patients with brain metastases. J Pain Symptom Manage 37(3):331–340
Claessens P, Menten J, Schotsmans P, Broeckaert B (2011) Development and validation of a modified version of the Edmonton Symptom Assessment Scale in a Flemish palliative care population. Am J Hosp Palliat Care 28(7):475–482
Chinda M, Jaturapatporn D, Kirshen AJ, Udomsubpayakul U (2011) Reliability and validity of a Thai version of the edmonton symptom assessment scale (ESAS-Thai). J Pain Symptom Manage 42(6):954–960
Carvajal A, Centeno C, Watson R, Bruera E (2011) A comprehensive study of psychometric properties of the Edmonton Symptom Assessment System (ESAS) in Spanish advanced cancer patients. Eur J Cancer 47(12):1863–1872
Guirimand F, Buyck J-F, Lauwers-Allot E, Revnik J, Kerguen T, Aegerter P et al (2010) Cancer-related symptom assessment in France: validation of the French M. D. Anderson Symptom Inventory. J Pain Symptom Manage 39(4):721–733
Yildirim Y, Tokem Y, Bozkurt N, Fadiloglu C, Uyar M, Uslu R (2011) Reliability and validity of the Turkish version of the Memorial Symptom Assessment Scale in cancer patients. Asian Pac J Cancer Prev 12(12):3389–3396
Badger TA, Segrin C, Meek P (2011) Development and validation of an instrument for rapidly assessing symptoms: the general symptom distress scale. J Pain Symptom Manage 41(3):535–548
Aoun SM, Monterosso L, Kristjanson LJ, McConigley R (2011) Measuring symptom distress in palliative care: psychometric properties of the Symptom Assessment Scale (SAS). J Palliat Med 14(3):315–321
Wang XS, Wang Y, Guo H, Mendoza TR, Hao XS, Cleeland CS (2004) Chinese version of the M. D. Anderson Symptom Inventory: validation and application of symptom measurement in cancer patients. Cancer 101(8):1890–1901
Mystakidou K, Cleeland C, Tsilika E, Katsouda E, Primikiri A, Parpa E et al (2004) Greek M.D. Anderson Symptom Inventory: validation and utility in cancer patients. Oncology 67(3-4):203–210
Okuyama T, Wang XS, Akechi T, Mendoza TR, Hosaka T, Cleeland CS et al (2003) Japanese version of the MD Anderson Symptom Inventory: a validation study. J Pain Symptom Manage 26(6):1093–1104
Abu-Saad Huijer H, Sagherian K, Tamim H (2014) Validation of the Arabic version of the memorial symptom assessment scale among Lebanese cancer patients. J Pain Symptom Manage
Kwon JH, Nam SH, Koh S, Hong YS, Lee KH, Shin SW et al (2013) Validation of the edmonton symptom assessment system in Korean patients with cancer. J Pain Symptom Manage 46(6):947–956
Harding G, Cella D, Robinson D Jr, Mahadevia PJ, Clark J, Revicki DA (2007) Symptom burden among patients with renal cell carcinoma (RCC): content for a symptom index. Health Qual Life Outcomes 5:34
Hickman SE, Tilden VP, Tolle SW (2001) Family reports of dying patients' distress: the adaptation of a research tool to assess global symptom distress in the last week of life. J Pain Symptom Manage 22(1):565–574
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67(6):361–370
Morrow GR (1984) Methodology in behavioral and psychosocial cancer research. The assessment of nausea and vomiting. Past problems, current issues and suggestions for future research. Cancer 53(10 Suppl):2267–2280
Sitzia J, Dikken C, Hughes J (1997) Psychometric evaluation of a questionnaire to document side-effects of chemotherapy. J Adv Nurs 25(5):999–1007
Rhodes VA, Watson PM, Johnson MH (1984) Development of reliable and valid measures of nausea and vomiting. Cancer Nurs 7(1):33–41
Barresi MJ, Shadbolt B, Byrne D, Stuart-Harris R (2003) The development of the Canberra symptom scorecard: a tool to monitor the physical symptoms of patients with advanced tumours. BMC Cancer 3:32
Chang VT, Hwang SS, Feuerman M, Kasimis BS, Thaler HT (2000) The memorial symptom assessment scale short form (MSAS-SF). Cancer 89(5):1162–1171
de Haes JC, van Knippenberg FC, Neijt JP (1990) Measuring psychological and physical distress in cancer patients: structure and application of the Rotterdam Symptom Checklist. Br J Cancer 62(6):1034–1038
Munro AJ, Potter S (1996) A quantitative approach to the distress caused by symptoms in patients treated with radical radiotherapy. Br J Cancer 74(4):640–647
Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Kiyasu E et al (1994) The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer 30A(9):1326–1336
Rhodes VA, McDaniel RW, Homan SS, Johnson M, Madsen R (2000) An instrument to measure symptom experience. Symptom occurrence and symptom distress. Cancer Nurs 23(1):49–54
Stein KD, Denniston M, Baker F, Dent M, Hann DM, Bushhouse S et al (2003) Validation of a modified Rotterdam Symptom Checklist for use with cancer patients in the United States. J Pain Symptom Manage 26(5):975–989
Watson M, ML, Maguire GP (1992) Further development of a quality of life measure for cancer patients: The Rotterdam Symptom Checklist (revised). Psychooncology 1:35-44
Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M et al (2000) Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer 89(7):1634–1646
Chow E, Wong R, Connolly R, Hruby G, Franzcr, Franssen E et al (2001) Prospective assessment of symptom palliation for patients attending a rapid response radiotherapy program. feasibility of telephone follow-up. J Pain Symptom Manage 22(2):649–656
McCorkle R, Quint-Benoliel J (1983) Symptom distress, current concerns and mood disturbance after diagnosis of life-threatening disease. Soc Sci Med 17(7):431–438
Holmes S (1991) Preliminary investigations of symptom distress in two cancer patient populations: evaluation of a measurement instrument. J Adv Nurs 16(4):439–446
Bruera E, MacDonal S (1993) Audit methods: the edmonton symptom assessment system. In: Higgenson I (ed) Clinical audit in palliative care, 1st edn. Radcliffe Medical Press, Oxford, pp 61–77
Morrow GR (1992) A patient report measure for the quantification of chemotherapy induced nausea and emesis: psychometric properties of the Morrow assessment of nausea and emesis (MANE). Br J Cancer Suppl 19:S72–S74
Slocumb EM, Cole FL (1991) A practical approach to content validation. Appl Nurs Res 4(4):192–195
Brown V, Sitzia J, Richardson A, Hughes J, Hannon H, Oakley C (2001) The development of the Chemotherapy Symptom Assessment Scale (C-SAS): a scale for the routine clinical assessment of the symptom experiences of patients receiving cytotoxic chemotherapy. Int J Nurs Stud 38(5):497–510
Youngblood M, Williams PD, Eyles H, Waring J, Runyon S (1994) A comparison of two methods of assessing cancer therapy-related symptoms. Cancer Nurs 17(1):37–44
Peruselli C, Camporesi E, Colombo AM, Cucci M, Mazzoni G, Paci E (1993) Quality-of-life assessment in a home care program for advanced cancer patients: a study using the Symptom Distress Scale. J Pain Symptom Manage 8(5):306–311
Bruera E, El Osta B, Valero V, Driver LC, Pei BL, Shen L et al (2007) Donepezil for cancer fatigue: a double-blind, randomized, placebo-controlled trial. J Clin Oncol 25(23):3475–3481
Bruera E, Valero V, Driver L, Shen L, Willey J, Zhang T et al (2006) Patient-controlled methylphenidate for cancer fatigue: a double-blind, randomized, placebo-controlled trial. J Clin Oncol 24(13):2073–2078
Ibbotson T, Maguire P, Selby P, Priestman T, Wallace L (1994) Screening for anxiety and depression in cancer patients: the effects of disease and treatment. Eur J Cancer 30A(1):37–40
Razavi D, Delvaux N, Farvacques C, Robaye E (1990) Screening for adjustment disorders and major depressive disorders in cancer in-patients. Br J Psychiatry 156:79–83
Lobchuk MM (2003) The memorial symptom assessment scale: modified for use in understanding family caregivers' perceptions of cancer patients' symptom experiences. J Pain Symptom Manage 26(1):644–654
Chang VT, Hwang SS, Kasimis B, Thaler HT (2004) Shorter symptom assessment instruments: the Condensed Memorial Symptom Assessment Scale (CMSAS). Cancer Invest 22(4):526–536
Nelson JE, Meier DE, Oei EJ, Nierman DM, Senzel RS, Manfredi PL et al (2001) Self-reported symptom experience of critically ill cancer patients receiving intensive care. Crit Care Med 29(2):277–282
Ewing G, Todd C, Rogers M, Barclay S, McCabe J, Martin A (2004) Validation of a symptom measure suitable for use among palliative care patients in the community: CAMPAS-R. J Pain Symptom Manage 27(4):287–299
Nunnally J, I. B (1994) Psychometric theory. Psychometric theory, McGraw-Hill, New York, p. 256
Chang VT, Hwang SS, Feuerman M (2000) Validation of the Edmonton symptom assessment scale. Cancer 88(9):2164–2171
Pautex S, Berger A, Chatelain C, Herrmann F, Zulian GB (2003) Symptom assessment in elderly cancer patients receiving palliative care. Crit Rev Oncol Hematol 47(3):281–286
Ewing G, Rogers M, Barclay S, McCabe J, Martin A, Campbell M et al (2006) Palliative care in primary care: a study to determine whether patients and professionals agree on symptoms. Br J Gen Pract 56(522):27–34
Hoekstra J, de Vos R, van Duijn NP, Schade E, Bindels PJ (2006) Using the symptom monitor in a randomized controlled trial: the effect on symptom prevalence and severity. J Pain Symptom Manage 31(1):22–30
Hoekstra J, Bindels PJ, van Duijn NP, Schade E (2004) The symptom monitor. A diary for monitoring physical symptoms for cancer patients in palliative care: feasibility, reliability and compliance. J Pain Symptom Manage 27(1):24–35
Richardson LA, Jones GW (2009) A review of the reliability and validity of the Edmonton Symptom Assessment System. Curr Oncol 16(1):55
Smith AB, Selby PJ, Velikova G, Stark D, Wright EP, Gould A et al (2002) Factor analysis of the Hospital Anxiety and Depression Scale from a large cancer population. Psychol Psychother 75(Pt 2):165–176
Moorey S, Greer S, Watson M, Gorman C, Rowden L, Tunmore R et al (1991) The factor structure and factor stability of the hospital anxiety and depression scale in patients with cancer. Br J Psychiatry 158:255–259
Vignaroli E, Pace EA, Willey J, Palmer JL, Zhang T, Bruera E (2006) The Edmonton Symptom Assessment System as a screening tool for depression and anxiety. J Palliat Med 9(2):296–303
Williams PD, Ducey KA, Sears AM, Williams AR, Tobin-Rumelhart SE, Bunde P (2001) Treatment type and symptom severity among oncology patients by self-report. Int J Nurs Stud 38(3):359–367
Baker F, Denniston M, Zabora J, Polland A, Dudley WN (2002) A POMS short form for cancer patients: psychometric and structural evaluation. Psychooncology 11(4):273–281
Reddy S, Bruera E, Pace E, Zhang K, Reyes-Gibby CC (2007) Clinically important improvement in the intensity of fatigue in patients with advanced cancer. J Palliat Med 10(5):1068–1075
Philip J, Smith WB, Craft P, Lickiss N (1998) Concurrent validity of the modified Edmonton Symptom Assessment System with the Rotterdam Symptom Checklist and the Brief Pain Inventory. Support Care Cancer 6(6):539–541
Lund I, Lundeberg T, Sandberg L, Budh CN, Kowalski J, Svensson E (2005) Lack of interchangeability between visual analogue and verbal rating pain scales: a cross sectional description of pain etiology groups. BMC Med Res Methodol 5:31
Huschka MM, Mandrekar SJ, Schaefer PL, Jett JR, Sloan JA (2007) A pooled analysis of quality of life measures and adverse events data in north central cancer treatment group lung cancer clinical trials. Cancer 109(4):787–795
Bland JM, Altman DG (1999) Measuring agreement in method comparison studies. Stat Methods Med Res 8(2):135–160
Schipper H, Clinch J, McMurray A, Levitt M (1984) Measuring the quality of life of cancer patients: the Functional Living Index-Cancer: development and validation. J Clin Oncol 2(5):472–483
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85(5):365–376
Huskisson E (1983) Visual analogue scales. In: Melzack R (ed) Pain management and assessment. Raven, New York, pp 33–37
Hinds PS, Schum L, Srivastava DK (2002) Is clinical relevance sometimes lost in summative scores? West J Nurs Res 24(4):345–353
Vickers AJ (2004) Statistical considerations for use of composite health-related quality-of-life scores in randomized trials. Qual Life Res 13(4):717–723
Watanabe S, Nekolaichuk C, Beaumont C, Mawani A (2009) The Edmonton symptom assessment system—what do patients think? Support Care Cancer 17(6):675–683
Holmes S (1989) Use of a modified symptom distress scale in assessment of the cancer patient. Int J Nurs Stud 26(1):69–79
Sutherland HJ, Walker P, Till JE (1988) The development of a method for determining oncology patients' emotional distress using linear analogue scales. Cancer Nurs 11(5):303–308
Morrow GR, Chiarello RJ, Derogatis LR (1978) A new scale for assessing patients' psychosocial adjustment to medical illness. Psychol Med 8(4):605–610
Pud D (2014) The psychometric properties of the Hebrew version of the Memorial Symptom Assessment Scale (MSAS-Heb) in breast cancer patients. J Pain Symptom Manage
Browall M, Kenne Sarenmalm E, Nasic S, Wengstrom Y, Gaston-Johansson F (2013) Validity and reliability of the Swedish version of the Memorial Symptom Assessment Scale (MSAS): an instrument for the evaluation of symptom prevalence, characteristics, and distress. J Pain Symptom Manage 46(1):131–141
McCorkle R, Young K (1978) Development of a symptom distress scale. Cancer Nurs 1(5):373–378
Chow E, Davis L, Holden L, Tsao M, Danjoux C (2005) Prospective assessment of patient-rated symptoms following whole brain radiotherapy for brain metastases. J Pain Symptom Manage 30(1):18–23
Chow E, Fan G, Hadi S, Filipczak L (2007) Symptom clusters in cancer patients with bone metastases. Support Care Cancer 15(9):1035–1043
Stromgren AS, Groenvold M, Pedersen L, Olsen AK, Sjogren P (2002) Symptomatology of cancer patients in palliative care: content validation of self-assessment questionnaires against medical records. Eur J Cancer 38(6):788–794
Gabrilove JL, Perez EA, Tomita DK, Rossi G, Cleeland CS (2007) Assessing symptom burden using the M. D. Anderson symptom inventory in patients with chemotherapy-induced anemia: results of a multicenter, open-label study (SURPASS) of patients treated with darbepoetin-alpha at a dose of 200 microg every 2 weeks. Cancer 110(7):1629–1640
Yamagishi A, Morita T, Miyashita M, Kimura F (2009) Symptom prevalence and longitudinal follow-up in cancer outpatients receiving chemotherapy. J Pain Symptom Manage 37(5):823–830
Chen ML, Tseng HC (2006) Symptom clusters in cancer patients. Support Care Cancer 14(8):825–830
Chen ML, Lin CC (2007) Cancer symptom clusters: a validation study. J Pain Symptom Manage 34(6):590–599
Acknowledgments
This paper was completed in part while one of the authors (D.W.) was a Lord Harris Visiting Fellow at the Tseu Medical Institute, Manchester Harris College, University of Oxford, UK, May–June 2011. We are grateful to Gretchen Hallerberg for help with the review of the literature. We thank Matthew Karafa, PhD; Wael Lasheen, MD; and Curtis Tatsuoka, PhD for their comments on the manuscript and Ellen Schleckman and Aditya Nair for their administrative assistance.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Jordanka Kirkova was supported in part by a fellowship grant in End-of-Life Care from the Mt. Sinai Health Care Foundation.
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Harry R. Horvitz Center is a World Health Organization Demonstration Project in Palliative Medicine and an ESMO Designated Center of Integrated Oncology and Palliative Care.
Rights and permissions
About this article
Cite this article
Aktas, A., Walsh, D. & Kirkova, J. The psychometric properties of cancer multisymptom assessment instruments: a clinical review. Support Care Cancer 23, 2189–2202 (2015). https://doi.org/10.1007/s00520-015-2732-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2732-7