Abstract
Purpose
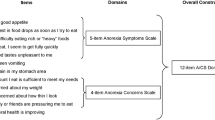
Patients with cancer anorexia-cachexia syndrome (CACS) suffer a significant symptom burden, impaired quality of life (QoL), and shorter survival. Measurement of QoL impairments related to CACS is thereby important both in clinical practice and in research. We aimed to further validate the Functional Assessment of Anorexia-Cachexia Therapy (FAACT) scale in an advanced lung cancer population.
Methods
We tested the performance of the FAACT and its anorexia-cachexia subscale (ACS) within a dataset of patients with advanced non-small cell lung cancer (aNSCLC), using standard statistical methods. We then compared the performance of commonly used QoL measures stratified by CACS status and by patient self-report of appetite and weight loss.
Results
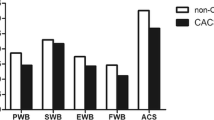
The FAACT and its ACS demonstrate internal validity consistent with acceptable published ranges for other QoL scales (Cronbach alpha = 0.9 and 0.79, respectively). Correlation coefficients demonstrate moderate correlations in the expected directions between FAACT and ACS and scales that measure related constructs. Comparing patients with and without CACS, the ACS is more sensitive to change than other QoL instruments (mean score 33.1 vs. 37.2, p = 0.011, ES = 0.58).
Conclusion
In patients with aNSCLC, the FAACT and its ACS performed well compared with other instruments, further supporting their validity and value in clinical research. FAACT and ACS scores covaried with symptoms and other QoL changes that are typical hallmarks of CACS, lending further support to their use as QoL endpoints in clinical trials among patients with CACS.
Similar content being viewed by others
References
Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD (2008) Cachexia: a new definition. Clin Nutr 27(6):793–799. doi:10.1016/j.clnu.2008.06.013
Fearon KC, Voss AC, Hustead DS, Cancer Cachexia Study G (2006) Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr 83(6):1345–1350
George J, Cannon T, Lai V, Richey L, Zanation A, Hayes DN, Shores C, Guttridge D, Couch M (2007) Cancer cachexia syndrome in head and neck cancer patients: part II. Pathophysiology. Head Neck 29(5):497–507. doi:10.1002/hed.20630
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12(5):489–495. doi:10.1016/S1470-2045(10)70218-7
Couch M, Lai V, Cannon T, Guttridge D, Zanation A, George J, Hayes DN, Zeisel S, Shores C (2007) Cancer cachexia syndrome in head and neck cancer patients: part I. Diagnosis, impact on quality of life and survival, and treatment. Head Neck 29(4):401–411. doi:10.1002/hed.20447
LeBlanc TW, Nipp RD, Rushing CN, Samsa GP, Locke SC, Kamal AH, Cella D, Abernethy AP (2014) Correlation between the International Consensus Definition of the Cancer Anorexia Cachexia Syndrome (CACS) and Patient-Centered Outcomes in Advanced Non-small Cell Lung Cancer. J Pain Symptom Manag. doi:10.1016/j.jpainsymman.2014.09.008
Cella DF, Tulsky DS (1993) Quality of life in cancer: definition, purpose, and method of measurement. Cancer Invest 11(3):327–336
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J et al (1993) The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol 11(3):570–579
Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P (1995) Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer 12(3):199–220
Ribaudo JM, Cella D, Hahn EA, Lloyd SR, Tchekmedyian NS, Von Roenn J, Leslie WT (2000) Re-validation and shortening of the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) questionnaire. Qual Life Res 9(10):1137–1146
Wheelwright S, Darlington AS, Hopkinson JB, Fitzsimmons D, White A, Johnson CD (2013) A systematic review of health-related quality of life instruments in patients with cancer cachexia. Support Care Cancer 21(9):2625–2636. doi:10.1007/s00520-013-1881-9
Butt Z, Webster K, Eisenstein AR, Beaumont J, Eton D, Masters GA, Cella D (2005) Quality of life in lung cancer: the validity and cross-cultural applicability of the Functional Assessment of Cancer Therapy-Lung scale. Hematol Oncol Clin North Am 19(2):389–420. doi:10.1016/j.hoc.2005.02.009, viii
Suh SY, Leblanc TW, Shelby RA, Samsa GP, Abernethy AP (2011) Longitudinal patient-reported performance status assessment in the cancer clinic is feasible and prognostic. J Oncol Pract 7(6):374–381. doi:10.1200/JOP.2011.000434
Abernethy AP, Currow DC (2010) Need for mechanistically focused research of global systemic interventions in palliative care. J Pain Symptom Manag 40(3):e5–e8. doi:10.1016/j.jpainsymman.2010.06.006
Temel J, Currow DC, Fearon K, Gleich L, Yan Y, Friend J, Abernethy AP (2014) Anamorelin for the treatment of cancer anorexia-cachexia in NSCLC: Results from the phase 3 studies ROMANA 1 and 2. Paper presented at the European Society of Medical Oncology, Madrid, 2014
Acknowledgments
Dr. Abernethy reports personal fees (as ownership or employment) from Advoset, Orange Leaf Associates, Athena Health and Flatiron Health, Inc. Grants from Alliance for Clinical Trials in Oncology, American Cancer Society, Bristol-Myers Squibb, Celgene, DARA, Denderon, GlaxoSmithKline, Helsinn Healthcare, Helsinn Therapeutics, Kanglaite, Mayo Clinic, Medical College of Wisconsin, Memorial Sloan Kettering Cancer Center, Pfizer, University of North Carolina at Chapel HIll, University of South Florida and federal grants from NIH, National Cancer Institute, AHRA, National Institute for Nursing Research, National Institutes on Aging; personal fees from American Academy of Hospice and Palliative Medicine (as immediate past president), Pfizer, and ACORN Research. Dr. LeBlanc is a recipient of a Junior Career Development Award from the National Palliative Care Research Center (NPCRC), has received research support (paid to Duke University Medical Center) from Celgene and Helsinn Therapeutics, and honoraria from Helsinn (<$5000). The authors acknowledge the editorial assistance of Donald T. Kirkendall, ELS, a Duke-employed medical editor.
Conflicts of interest
The remaining co-authors have no disclosures or conflicts of interest to report.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was funded in part by a research grant from Helsinn to Duke University Medical Center.
Rights and permissions
About this article
Cite this article
LeBlanc, T.W., Samsa, G.P., Wolf, S.P. et al. Validation and real-world assessment of the Functional Assessment of Anorexia-Cachexia Therapy (FAACT) scale in patients with advanced non-small cell lung cancer and the cancer anorexia-cachexia syndrome (CACS). Support Care Cancer 23, 2341–2347 (2015). https://doi.org/10.1007/s00520-015-2606-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2606-z