Abstract
Purpose
This trial assessed the ability to enhance health-related quality of life (HRQL) and patient-reported outcome (PRO) evaluation in trials and patient management using computer assistance with a handheld device, called a personal digital assistant. The study assessed ease of use and psychometric properties of this approach, comparing the Lung Cancer Symptom Scale (LCSS) paper form with the electronic (eLCSS-QL). Objectives were to: (1) measure completion times; (2) evaluate acceptability by patients, nurses, and physicians; (3) determine the correlation of the eLCSS-QL with the paper version; and (4) determine the feasibility of using a shorter visual analogue scale (VAS) in the electronic version.
Patients and methods
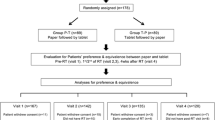
Patients were entered at 12 COMET clinics. All had: (a) stage III or IV non-small cell lung cancer, (b) Karnofsky performance status (KPS) ≥ 60, (c) no prior chemotherapy, and (d) received initial courses of docetaxel + platinum. Of the148 patients enrolled, characteristics were: men, 57 %; median, KPS 80 %; and median age, 67 years. Of these, 131 patients completed the evaluation form.
Results
The eLCSS-QL had excellent acceptance by patients, nurses, and physicians. Patients required 2.2 min (mean) to complete the eLCSS-QL. Reliability coefficients using Cronbach’s alpha were high for the paper (0.84) and electronic (0.88) versions. The correlation coefficient between forms was high (0.92). The length of the VAS on the handheld pc (53 mm versus 100 mm on the paper format) resulted in nearly identical scores.
Conclusions
The high acceptance rate by patients and professionals, the rapid completion time, ease of use, and strong psychometric properties confirm that the electronic LCSS (eLCSS-QL) is practical for use in trials and patient management. This study indicates that computer assistance helps overcome barriers associated with evaluating HRQL and PROs.

Similar content being viewed by others
References
Lipscomb J, Gotay CC, Snyder CF (2007) Patient-reported outcomes in cancer: a review of recent research and policy initiatives. CA Cancer J Clin 57:278–300
Morris J, Perez D, McNoe B (1998) The use of quality of life data in clinical practice. Qual Life Res 7:85–91
Higginson IJ, Carr AJ (2001) Using quality of life measures in the clinical setting. B Med J 322:1297–1300
Passik SD, Kirsh KL (2000) The importance of quality-of-life endpoints in clinical trials to the practicing oncologist. Hematol Oncol Clin North Am 14:877–886
Hollen PJ, Gralla RJ, Kris MG, Potanovich LM (1993) Quality of life assessment in individuals with lung cancer: testing the Lung Cancer Symptom Scale (LCSS). Eur J Cancer 29A:S51–S58
Hollen PJ, Gralla RJ, Kris MG, Cox C, Belani CP, Grunberg SM, Crawford J, Neidhart JA (1994) Measurement of quality of life in patients with lung cancer in multicenter trials of new therapies: psychometric assessment of the Lung Cancer Symptom Scale. Cancer 73:2087–2098
Nunnally JC, Bernstein IH (1994) Psychometric theory, 3rd edn. McGraw-Hill, New York
Bergman B, Aaronson NK, Ahmedzai S et al (1994) The EORTC QLQ-LC13: a modular supplement to the EORTC core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. Eur J Cancer 30A(5):635–642
Cella DF, Bonomi AE, Lloyd SR et al (1995) Reliability and validity of the Functional Assessment of Cancer Therapy—Lung (FACT-L) quality of life instrument. Lung Cancer 12:199–220
Earle CC, Weeks JC (2005) The science of quality-of-life measurement in lung cancer. In: Lipscomb J, Gotay CC, Synder C (eds) Outcomes assessment in cancer: measures, method, and applications. Cambridge University Press, Cambridge, p 171
Fossella F, Pereira JR, von Pawel J et al (2003) Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol 21:3016–3024
Hollen PJ, Gralla RJ, Kris MG, Cox C (1994) Quality of life during clinical trials: conceptual model for the Lung Cancer Symptom Scale (LCSS). Support Care Cancer 2:213–222
Hollen PJ, Gralla RJ, Kris MG, Eberly SW, Cox C (1999) Normative data and trends in quality of life from the Lung Cancer Symptom Scale (LCSS). Support Care Cancer 7:140–148
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1(8476):307–310
Shrout PE, Fleiss JL (1979) Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86(2):420–428
Lin L (1989) A concordance correlation coefficient to evaluate reproducibility. Biometrics 45:255–268
Hollen PJ, Gralla RJ, Kris MG, McCoy S, Donaldson GW, Moinpour CM (2005) A comparison of visual analogue and numerical rating scale formats for the Lung Cancer Symptom Scale: does format affect patient ratings of symptoms and quality of life? Qual Life Res 14:837–847
Kraemer HC, Thiemann S (1987) How many subjects? Statistical power analysis in research. Sage, Newbury Park
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Hollen PJ, Gralla RJ, Rittenberg CN (2004) Quality of life as a clinical trial endpoint: determining the appropriate interval for repeated assessments in patients with advanced lung cancer. Support Care Cancer 12:767–773
Hollen PJ, Gralla RJ, Symanowski JT, Liepa AM, Bizette GA (2004) Determining the frequency of quality of life assessment in chemotherapy treatment: using the LCSS-Meso in the randomized pemetrexed + cisplatin trial in 448 patients with mesothelioma as an example. J Clin Oncol 22(14S):8125
Acknowledgments
The following investigators and study nurses are acknowledged for their help with this study: Philip Kuruvilla MD; Henry Solow MD; Labib Zibdawi, MD; David Walde, MD; Sandeep R. Sedhev, MD; Bryan Pressnail, MD; John A. P. Gapski, MD, and Roger Levesque, MD. Arlene Welch; Laura Tindall; Clara Peters-Onagoruwa; Valerie Butta; Cindy John; Lynda Phippard; Karen Hopper; Pat Champagne; Nasreen DeYoe; Nathalie Kovacevich; Heather Kimber; Nancy Doyle; Kim Marsh-Gray; Marilyn Leighton; Jane Palmateer; and Alexandra Salvarrey. The authors wish to acknowledge the contributions of Karine Alloul and Benoit Cossette as well as many others working for Sanofi-Aventis Canada who helped throughout the conduct of the study. In addition, the authors acknowledge the technical support of Michael Bird.
Funding
Supported in part by a grant from Sanofi-Aventis Canada, Laval, QC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hollen, P.J., Gralla, R.J., Stewart, J.A. et al. Can a computerized format replace a paper form in PRO and HRQL evaluation? Psychometric testing of the computer-assisted LCSS instrument (eLCSS-QL). Support Care Cancer 21, 165–172 (2013). https://doi.org/10.1007/s00520-012-1507-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-012-1507-7