Abstract
Purpose
The purpose of this study was to assess the validity and reliability of the Korean version of the EQ-5D health questionnaire for use in patients with cancer in Korea.
Methods
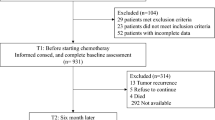
Patients with colorectal cancer were recruited from one ambulatory cancer center. Each participant consecutively self-administered the EQ-5D, the EORTC QLQ-C30, and the Short Form-36 (SF-36). Discriminatory ability was evaluated by comparing the SF-36 subscales with their corresponding EQ-5D dimensions. Convergent validity was assessed by examining the correlations between the EQ-5D index, EORTC QLQ-C30 subscales, and SF-36 scale and summary scores. Test-retest reliability was also evaluated.
Results
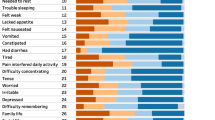
Subjects reporting problems in each EQ-5D dimension showed lower scores on all SF-36 subscales. As expected, the relationships were stronger between the EQ-5D functional dimensions and physical function on the EORTC QLQ-C30 and between the EQ-5D anxiety/depression dimension and emotional function on the EORTC QLQ-C30. The EQ-5D index and SF-36 scales were moderately or highly correlated. intraclass correlation coefficient of the EQ-5D index was 0.45.
Conclusions
The Korean version of the EQ-5D may be a valid tool for assessing the health-related quality of life of patients with cancer. However, further research is needed to determine the reliability of the Korean EQ-5D over different time intervals and disease conditions.
Similar content being viewed by others
References
The Korea Central Cancer Registry, National Cancer Center (2010) Annual report of cancer statistics in Korea in 2008. Ministry of Health and Welfare
Nayfield SG, Ganz PA, Moinpour CM et al (1992) Report from a National Cancer Institute (USA) workshop on quality of life assessment in cancer clinical trials. Qual Life Res 1(3):203–210
Torrance GW (1986) Measurement of health state utilities for economic appraisal. J Health Econ 5(1):1–30
Cost-effectiveness in health and medicine. In Gold MR, Siegal JE, Russell LB, et al (Eds.) (1996) Oxford University Press. New York
Räsänen P, Roine E, Sintonen H et al (2006) Use of quality-adjusted life years for the estimation of effectiveness of health care: a systematic literature review. Int J Technol Assess Health Care 22(2):235–41
Aaronson NK, Ahmedzai S, Bergman B et al (1993) The European Organization for Research and Treatment of Cancer QLQ C-30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–76
Rowen D, Brazier J, Young T et al (2011) Deriving a preference-based measure for cancer using the EORTC QLQ-C30. Value Health 14(5):721–31
(1996) Quality of life and pharmacoeconomics in clinical trials. 2nd edn. In Spilker, B. (Ed.) Lippincott-Raven. Philadelphia
Health Insurance Review and Assessment service (2006) Guidelines for economic evaluation of pharmaceuticals
Kim MH, Cho YS, Uhm WS et al (2005) Cross-cultural adaptation and validation of the Korean version of the EQ-5D in patients with rheumatic diseases. Qual Life Res 14(5):1401–1406
Kim SH, Kim HJ, Lee SI, et al. Comparing the psychometric properties of the EQ-5D-3L and EQ-5D-5L in cancer patients in Korea. Qual Life Res. 2011 Sep 23. DOI 10.1007/s11136-011-0018-1
Brooks R, Rabin RE, de Charro F (2003) The measurement and valuation of health status using EQ-5D: a European perspective. Kluwer Academic Publishers, Dordrecht
Yun YH, Park YS, Lee ES et al (2004) Validation of the Korean version of the EORTC QLQ-C30. Qual Life Res 13(4):863–868
Ware JE Jr, Kosinski MB, Bjorner JB et al (2007) User's manual for the SF-36v2® health survey, 2nd edn. Quality Metric Incorporated, Lincoln
Han CW, Lee EJ, Iwaya T et al (2004) Development of the Korean version of short-form 36-item health survey: health related QOL of healthy elderly people and elderly patients in Korea. Tohoku J Exp Med 203(3):189–194
McHorney CA, Ware JE Jr, Raczek AE (1993) The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 31(3):247–263
Brazier JE, Harper R, Jones NM et al (1992) Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ 305(6846):160–164
McHorney CA, Ware JE Jr, Lu JF et al (1994) The MOS 36-item short-form health survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care 32(1):40–66
Lee YK, Nam HS, Chuang LH et al (2009) South Korean time trade-off values for EQ-5D health states: modeling with observed values for 101 health states. Value Health 12(8):1187–1193
Brazier J, Roberts J, Deverill M (2002) The estimation of a preference-based measure of health from the SF-36. J Health Econ 21(2):271–292
Brazier J, Jones N, Kind P (1993) Testing the validity of the Euroqol and comparing it with the SF-36 health survey questionnaire. Qual Life Res 2(3):169–180
Kontodimopoulos N, Pappa E, Niakas D et al (2008) Validity of the EuroQoL (EQ-5D) instrument in a Greek general population. Value Health 11(7):1162–1169
Chang TJ, Tarn YH, Hsieh CL et al (2007) Taiwanese version of the EQ-5D: validation in a representative sample of the Taiwanese population. J Formos Med Assoc 106(12):1023–1031
Johnson JA, Coons SJ (1998) Comparison of the EQ-5D and SF-12 in an adult US sample. Qual Life Res 7(2):155–166
Johnson JA, Pickard AS (2000) Comparison of the EQ-5D and SF-12 health surveys in a general population survey in Alberta, Canada. Med Care 38(1):115–121
Lang HC, Chuang L, Shun SC et al (2010) Validation of EQ-5D in patients with cervical cancer in Taiwan. Support Care Cancer 18(10):1279–1286
Fleiss JL (1981) Statistical methods for rates and proportions, 2nd edn. Wiley, New York
Hripcsak G, Heitjan DF (2002) Measuring agreement in medical informatics reliability studies. J Biomed Inform 35(2):99–110
Hurst NP, Kind P, Ruta D et al (1997) Measuring health-related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQol (EQ-5D). Br J Rheumatol 36(5):551–559
Disclosures
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, S.H., Hwang, J.S., Kim, T.W. et al. Validity and reliability of the EQ-5D for cancer patients in Korea. Support Care Cancer 20, 3155–3160 (2012). https://doi.org/10.1007/s00520-012-1457-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-012-1457-0