Abstract
Background
The multikinase inhibitor sunitinib has enhanced the treatment of renal cell carcinoma and gastrointestinal stromal tumor through an improved clinical response with decreased systemic toxicities. However, sunitinib is frequently associated with dermatological adverse reactions. The physical and psychosocial impact of frequent dermatological toxicities can affect consistent antineoplastic therapy and quality of life.
Patients and methods
Dermatological adverse reaction information was compiled from Pfizer Medical Information and from abstracts from the 2007 American Society of Clinical Oncology annual meeting, Prostate Cancer Symposium, and Gastrointestinal Cancers Symposium. Published clinical trials of sunitinib in MEDLINE, Cochrane Library, Cochrane Controlled Trials Register, and EMBASE Drugs and Pharmacology databases were also included. Information was accessed on or before June 30, 2007.
Results
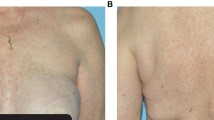
In the pooled analysis, all-grade hand–foot skin reaction occurred in 19% of patients (5% grades 3–4), skin discoloration in 28% (0% grades 3–4), dry skin in 16% (1% grades 3–4), skin rash in 13% (1% grades 3–4), dermatitis in 8% (2% grades 3–4), hair color changes in 10% (0% grades 3–4), alopecia in 6% (0% grades 3–4), and phototoxicity in <0.1%.
Conclusions
Dermatological reactions associated with sunitinib occur frequently. Evidence-based treatment recommendations are needed in order to maximize quality of life and optimize clinical outcome.

Similar content being viewed by others
References
Azad A, Annunziata C, Barrett T, Chen C, Steinberg S, Kwitkowski VE, McNally D, Kotz H, Minasian L, Kohn EC (2007) Dual targeting of vascular endothelial growth factor (VEGF) with sorafenib and bevacizumab: clinical and translational results. J Clin Oncol, ASCO Annual Meeting Proceedings Part I 25(18S):3542
Bang Y, Kang Y, Kang W, Boku N, Chung H, Lanzalone S, Lechuga MJ, Sherman L, Chao R, Sobrero A (2007) Sunitinib as second-line treatment for advanced gastric cancer: preliminary results from a phase II study. J Clin Oncol 25:4603
Bello CL, Sherman L, Zhou J, Verkh L, Smeraglia J, Mount J, Klamerus KJ (2006) Effect of food on the pharmacokinetics of sunitinib malate (SU11248), a multi-targeted receptor tyrosine kinase inhibitor: results from a phase I study in healthy subjects. Anticancer Drugs 17:353–358
Bhojani N, Jeldres C, Patard JJ, Perrotte P, Suardi N, Hutterer G, Patenaude F, Oudard S, Karakiewicz PI, (2007) Toxicities associated with the administration of sorafenib, sunitinib, and temsirolimus and their management in patients with metastatic renal cell carcinoma. Eur Urol DOI 10.1016/j.euroro.2007.11.037, Nov 26 [Epub ahead of print]
Botchkareva NV, Khlgatian M, Longley BJ, Botchkarev VA, Gilchrest BA (2001) SCF/c-kit signaling is required for cyclic regeneration of the hair pigmentation unit. Faseb J 15:645–658
Brazzelli V, Prestinari F, Barbagallo T, Rona C, Orlandi E, Passamonti F, Locatelli F, Zecca M, Villani S, Borroni G (2007) A long-term time course of colorimetric assessment of the effects of imatinib mesylate on skin pigmentation: a study of five patients. J Eur Acad Dermatol Venereol 21:384–387
Chow LQ, Eckhardt SG (2007) Sunitinib: from rational design to clinical efficacy. J Clin Oncol 25:884–896
Chu D, Lacouture ME, Fillos T, Wu S (2007) Risk of hand–foot skin reaction with sorafenib: a systematic review and meta-analysis. Acta Oncol 47(2):176–186
Cooney MM, Garcia J, Brell J, Dreicer R, Beatty K, Mekhail T, Bukowski R, Zwiebel J, Remick SC, Rini BI (2007) A phase I study of bevacizumab in combination with sunitinib in advanced solid tumors. J Clin Oncol 25:15532
Dancey J, Sausville EA (2003) Issues and progress with protein kinase inhibitors for cancer treatment. Nat Rev 2:296–313
Dasanu CA, Alexandrescu DT, Dutcher J (2007) Yellow skin discoloration associated with sorafenib use for treatment of metastatic renal cell carcinoma. South Med J 100:328–330
Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA, Desai J, Fletcher CD, George S, Bello CL, Huang X, Baum CM, Casali PG (2006) Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 368:1329–1338
Donner A, Klar N (1994) Methods for comparing event rates in intervention studies when the unit of allocation is a cluster. Am J Epidemiol 140:279–289
Erber R, Thurnher A, Katsen AD, Groth G, Kerger H, Hammes HP, Menger MD, Ullrich A, Vajkoczy P (2004) Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. Faseb J 18:338–340
Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, Armand JP, Scigalla P, Raymond E (2006) Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 24:25–35
Feldman DR, Kondagunta GV, Ronnen EA, Fischer P, Chang R, Baum M, Ginsberg MS, Ishill N, Patil S, Motzer RJ (2007) Phase I trial of bevacizumab plus sunitinib in patients (pts) with metastatic renal cell carcinoma (mRCC). J Clin Oncol 25:1
Fiedler W, Serve H, Dohner H, Schwittay M, Ottmann OG, O'Farrell AM, Bello CL, Allred R, Manning WC, Cherrington JM, Louie SG, Hong W, Brega NM, Massimini G, Scigalla P, Berdel WE, Hossfeld DK (2005) A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood 105:986–993
Gallagher DJ, Milowsky MI, Gerst SR, Iasonos A, Riches J, Boyle MG, Bajorin DF (2007) Phase II study of sunitinib in patients (pts) with relapsed or refractory urothelial carcinoma (UC). J Clin Oncol 25:5080
George S, Blay JY, Casali PG, Cesne AL, Morgan JA, Pokela J, Quigley MT, Tassell V, Baum CM, Demetri GD (2007) Continuous daily dosing (CDD) of sunitinib malate (SU) compares favorably with intermittent dosing in pts with advanced GIST. J Clin Oncol 25:10015
Gore ME, Porta C, Ourdard S, Bjamason G, Castellano D, Szczylik C, Mainwaring PN, Schoffski P, Rini BI, Bulkowski RM (2007) Sunitinib in metastatic renal cell carcinoma (mRCC): preliminary assessment of toxicity in an expanded access trial with subpopulation analysis. J Clin Oncol 25:1
Hagemann I, Proksch E (1996) Topical treatment by urea reduces epidermal hyperproliferation and induces differentiation in psoriasis. Acta Derm Venereol 76:353–356
Kondagunta GV, Hudes GR, Figlin R, Wilding G, Hariharan S, Kempin SN, Fayyad R, Hoosen S, Motzer RJ (2007) Sunitinib malate (SU) plus interferon (IFN) in first line metastatic renal cell cancer (mRCC): results of a dose-finding study. J Clin Oncol 25:5101
Loden M (2003) Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol 4:771–788
Luo D, Chen H, Searles G, Jimbow K (1995) Coordinated mRNA expression of c-Kit with tyrosinase and TRP-1 in melanin pigmentation of normal and malignant human melanocytes and transient activation of tyrosinase by Kit/SCF-R. Melanoma Res 5:303–309
Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, Ginsberg MS, Kim ST, Baum CM, DePrimo SE, Li JZ, Bello CL, Theuer CP, George DJ, Rini BI (2006) Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol 24:16–24
Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, Redman BG, Margolin KA, Merchan JR, Wilding G, Ginsberg MS, Bacik J, Kim ST, Baum CM, Michaelson MD (2006) Sunitinib in patients with metastatic renal cell carcinoma. JAMA 295:2516–2524
Motzer RJ, Figlin RA, Hutson TE, Tomczak P, Bukowski RM, Rixe O, Bjarnason GA, Kim ST, Chen I, Michaelson D (2007) Sunitinib versus interferon-alfa (IFN-α) as first-line treatment of metastatic renal cell carcinoma (mRCC): updated results and analysis of prognostic factors. J Clin Oncol 25:5024
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356:115–124
Mrozek-Orlowski ME, Frye DK, Sanborn HM (1999) Capecitabine: nursing implications of a new oral chemotherapeutic agent. Oncol Nurs Forum 26:753–762
Perez-Soler R, Saltz L (2005) Cutaneous adverse effects with HER1/EGFR-targeted agents: is there a silver lining? J Clin Oncol 23:5235–5246
Reichardt P, Yang YK, Ruka W, Seddon B, Baum CM, Demetri GD (2007) Subpopulation analyses in a worldwide treatment-use of sunitinib (SU) in GIST patients (pts) with resistance intolerance to prior imatinib (IM) therapy. J Clin Oncol 25:10022
Rixe O, Billemont B, Izzedine H (2007) Hypertension as a predictive factor of sunitinib activity. Ann Oncol 18:1117
Robert C, Faivre S, Raymond E, Armand JP, Escudier B (2005) Subungual splinter hemorrhages: a clinical window to inhibition of vascular endothelial growth factor receptors? Ann Intern Med 143:313–314
Robert C, Soria JC, Spatz A, Le Cesne A, Malka D, Pautier P, Wechsler J, Lhomme C, Escudier B, Boige V, Armand JP, Le Chevalier T (2005) Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol 6:491–500
Rock EP, Goodman V, Jiang JX, Mahjoob K, Verbois SL, Morse D, Dagher R, Justice R, Pazdur R (2007) Food and Drug Administration drug approval summary: sunitinib malate for the treatment of gastrointestinal stromal tumor and advanced renal cell carcinoma. Oncologist 12:107–113
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabarbara P, Seymour L (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353:123–132
Srinivas S, Roigas J, Gillessen S, Hamenberg U, Mulder PHD, Fountzilas G, Vogelzang N, Peschel C, Flodgren P, Escudier B (2007) Continuous daily administration of sunitinib in patients (pts) with cytokine-refractory metastatic renal cell carcinoma (mRCC): updated results. J Clin Oncol 25:5040
Strumberg D, Clark JW, Awada A, Moore MJ, Richly H, Hendlisz A, Hirte HW, Eder JP, Lenz HJ, Schwartz B (2007) Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist 12:426–437
Sweeney C, Verschraegen C, Chiorean C, Lee F, Jones S, Tye L, Bello A, Chao R, Burris H (2007) A phase I dose escalation study of sunitinib plus capecitabine in patients with advanced solid tumors. J Clin Oncol 25:3592
Tsai KY, Yang CH, Kuo TT, Hong HS, Chang JW (2006) Hand–foot syndrome and seborrheic dermatitis-like rash induced by sunitinib in a patient with advanced renal cell carcinoma. J Clin Oncol 24:5786–5788
Wood LS (2006) Nursing management: managing the side effects of sorafenib and sunitinib. Community Oncol 3:5
Zhu AX, Sahani DV, Tomaso ED, Duda D, Sindhwani V, Yoon SS, Blaszkowsky LS, Clark JW, Ryan DP, Jain RK (2007) A phase II study of sunitinib in patients with advanced hepatocellular cancer. J Clin Oncol 25:4637
Zurita AJ, Shore N, Kozloff M, Ryan CW, Beer TM, Maneval EC, Chen I, Logothetis C, (2007) Phase I study of sunitinib in combination with docetaxel and prednisone in patients (pts) with metastatic hormone refractory prostate cancer (mHPRC). In 2007 ASCO Prostate Cancer Symposium. Abstract No. 230
Acknowledgments
We thank Alfred Rademaker for the statistical analysis. M.E.L. is supported by a Zell Scholarship from the Robert H Lurie Cancer Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosenbaum, S.E., Wu, S., Newman, M.A. et al. Dermatological reactions to the multitargeted tyrosine kinase inhibitor sunitinib. Support Care Cancer 16, 557–566 (2008). https://doi.org/10.1007/s00520-008-0409-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-008-0409-1