Abstract
Objective
The aim of the study was to assess levels of chemotherapy-induced nausea and vomiting (CINV) in routine practice.
Materials and methods
The study was an observational prospective evaluation using patient self-reports. One hundred and two patients with cancer in a single cancer centre in UK receiving their first chemotherapy treatment participated in the study and were followed up over four cycles, providing a total of 272 assessments of nausea and vomiting. Data was collected with the use of the MASCC Antiemesis Tool (MAT), which is an eight-item short clinical scale assessing acute and delayed nausea and vomiting after chemotherapy.
Results
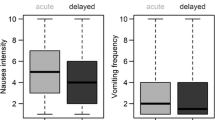
Results indicated that acute vomiting was experienced by 15.7% of the patients in cycle 1 and delayed vomiting by 14.7%, while acute nausea was present in 37.3% of the patients and delayed nausea in 47.1%, increasing over the subsequent cycles. Moderately emetogenic and highly emetogenic chemotherapy had the highest incidence of CINV, whereas patients receiving highly emetogenic chemotherapy showed significant levels of delayed nausea. Acute symptoms were more easily controlled than delayed symptoms.
Discussion
The data suggest that, while vomiting is well controlled, nausea remains a significant problem in practice, and optimal management of CINV is yet to be achieved. Understanding more clearly the biological basis of nausea will assist in managing this complex symptom more effectively in practice.
Similar content being viewed by others
References
Roscoe JA, Morrow GR, Hickok JT et al (2000) Nausea and vomiting remain a significant clinical problem: trends over time in controlling chemotherapy-induced nausea and vomiting in 1413 patients treated in the community. J Pain Symptom Manage 20:113–121
Hickok JT, Roscoe JA, Morrow GR et al (2003) Nausea and emesis remain significant problems of chemotherapy despite prophylaxis with 5-hydroxytryptamine-3 antiemesis: a University of Rochester James P. Wilmot Cancer center community clinical oncology program study of 360 cancer patients treated in the community. Cancer 97:2880–2886
De Boer-Dennert M, de Wit R, Schmitz PI, Djontono J et al (1997) Patient perceptions of the side-effects of chemotherapy: the influence of 5HT3 antagonists. Br J Cancer 76:1055–1061
Griffin AM, Butow PN, Coates AS et al (1996) On the receiving end. V: Patient perceptions of the side effects of cancer chemotherapy in 1993. Ann Oncol 7:189–195
Osoba D, Zee B, Warr D et al (1997) Effect of postchemotherapy nausea and vomiting on health-related quality of life. Support Care Cancer 5:307–313
Rusthoven JJ, Osoba D, Butts CA et al (1998) The impact of postchemotherapy nausea and vomiting on quality of life after moderately emetogenic chemotherapy. Support Care Cancer 6:389–395
Bloechl-Daum B, Deuson RR, Mavros P et al (2006) Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol 24:4472–4478
Foubert J, Vaessen G (2005) Nausea: the neglected symptom? Eur J Oncol Nurs 9:21–32
Bergkvist K, Wengstrom Y (2006) Symptom experiences during chemotherapy treatment—with focus on nausea and vomiting. Eur J Oncol Nurs 10:21–29
Glaus A, Knipping C, Morant R, Böhme C, Lebert B, Glawogger B, Fernandez Ortega P, Hüsler A, Deuson R (2004) Chemotherapy-induced nausea and vomiting in routine practice: a European perspective. Support Care Cancer 12:708–715
Ihbe-Heffinger A, Ehlken B, Bernard R et al (2004) The impact of delayed chemotherapy-induced nausea and vomiting on patients, health resource utilization and costs in German cancer centers. Ann Oncol 15:526–536
Molassiotis A (2005) Managing nausea and vomiting after cancer treatments: patients still suffer unnecessarily. Eur J Oncol Nurs 9:4–5
Tonato M, Clark-Snow R, Osoba D et al (2005) Emesis induced by low or minimal emetic risk chemotherapy. Support Care Cancer 13:109–111
Molassiotis A, Coventry PA, Stricker CT, Clements C, Eaby B, Velders L, Rittenberg C, Gralla RJ (2007) Validation and psychometric properties of a short clinical scale to measure chemotherapy-induced nausea and vomiting: the MASCC Antiemesis Tool (MAT). J Pain Symptom Manage 34:148–159
Hesketh PJ (1999) Defining the emetogenicity of cancer chemotherapy regimens: relevance to clinical practice. Oncologist 4:191–196
Grunberg SM, Osoba D, Hesketh PJ et al (2005) Evaluation of new antiemetic agents and definition of antineoplastic agent emetogenicity-an update. Support Care Cancer 13:80–84
Kris MG, Hesketh PJ, Somerfield MR et al (2006) American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol 24:2932–2947
Cabana MD, Rand CS, Powe NR et al (1999) Why don’t physicians follow practice guidelines? A framework for improvement. J Am Med Assoc 282:1458–1465
Hesketh PJ, Grunberg SM, Gralla RJ et al (2003) The oral neurokinin-1 antagonist Aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin—the Aprepitant protocol 052 study group. J Clin Oncol 21:4112–4119
Poli-Bigelli S, Rodrigues-Pereira J, Carides AD et al (2003) Addition of the neurokinin 1 receptor antagonist Aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer 97:3090–3098
Roila F, Donati D, Tamberi S, Margutti G (2002) Delayed emesis: incidence, pattern, prognostic factors and optimal treatment. Support Care Cancer 10:88–95
Aapro MS, Molassiotis A, Olver I (2005) Anticipatory nausea and vomiting. Support Care Cancer 13:117–121
Molassiotis A, Yung HP, Yam BMC, Chan FYS, Mok TSK (2002) The effectiveness of progressive muscle relaxation training in managing chemotherapy-induced nausea and vomiting in Chinese breast cancer patients: a randomized controlled trial. Support Care Cancer 10:237–246
Molassiotis A, Helin AM, Dabbour R, Hummerston S (2007) The effects of P6 acupressure in the prophylaxis of chemotherapy-related nausea and vomiting in breast cancer patients. Complem Ther Med 15:3–12
Morrow GR, Lindke J, Black PM (1991) Predicting the development of anticipatory nausea in cancer patients: prospective evaluation of eight clinical characteristics. J Pain Symptom Manage 6:215–223
Gralla RJ, Osoba D, Kris MG, Kirkbride P, Hesketh PJ, Chinnery LW, Clark-Snow R, Gill DP, Groshen S, Grunberg S, Koeller JM, Morrow GR, Perez EA, Silber JH, Pfister DG (1999) Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. American Society of Clinical Oncology. J Clin Oncol 17:2971–2994
Fabi A, Barduagni M, Lauro S et al (2003) Is delayed chemotherapy-induced emesis well managed in oncological clinical practice? An observational study. Support Care Cancer 11:156–161
Grunberg SM (2004) Chemotherapy-induced nausea and vomiting: prevention, detection, and treatment—how are we doing? J Support Oncol 2(Suppl 1):1–10
Acknowledgements
The study was funded by an unrestricted grant from Merck.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Molassiotis, A., Saunders, M.P., Valle, J. et al. A prospective observational study of chemotherapy-related nausea and vomiting in routine practice in a UK cancer centre. Support Care Cancer 16, 201–208 (2008). https://doi.org/10.1007/s00520-007-0343-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-007-0343-7