Abstract
Background and objectives
Chronic kidney disease is a persistent chronic health condition commonly seen in pediatric nephrology programs. Our study aims to evaluate the sensitivity of the Patient Reported Outcomes Measurement Information System (PROMIS) pediatric instrument to indicators of disease severity and activity in pediatric chronic kidney disease.
Methods
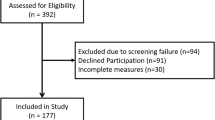
This cross sectional study included 233 children 8–17 years old, with chronic kidney disease from 16 participating institutions in North America. Disease activity indicators, including hospitalization in the previous 6 months, edema, and number of medications consumed daily, as well as disease severity indicators of kidney function and coexisting medical conditions were captured. PROMIS domains, including depression, anxiety, social-peer relationships, pain interference, fatigue, mobility, and upper extremity function, were administered via web-based questionnaires. Absolute effect sizes (AES) were generated to demonstrate the impact of disease on domain scores. Four children were excluded because of missing glomerular filtration rate (GFR) estimations.
Results
Of the 229 children included in the final analysis, 221 completed the entire PROMIS questionnaire. Unadjusted PROMIS domains were responsive to chronic kidney disease activity indicators and number of coexisting conditions. PROMIS domain scores were worse in the presence of recent hospitalizations (depression AES 0.33, anxiety AES 0.42, pain interference AES 0.46, fatigue AES 0.50, mobility AES 0.49), edema (depression AES 0.50, anxiety AES 0.60, pain interference AES 0.77, mobility AES 0.54) and coexisting medical conditions (social peer-relationships AES 0.66, fatigue AES 0.83, mobility AES 0.60, upper extremity function AES 0.48).
Conclusions
The PROMIS pediatric domains of depression, anxiety, social-peer relationships, pain interference, and mobility were sensitive to the clinical status of children with chronic kidney disease in this multi-center cross sectional study. We demonstrated that a number of important clinical characteristics including recent history of hospitalization and edema, affected patient perceptions of depression, anxiety, pain interference, fatigue and mobility. The PROMIS instruments provide a potentially valuable tool to study the impact of chronic kidney disease. Additional studies will be required to assess responsiveness in PROMIS score with changes in disease status over time.

Similar content being viewed by others
References
Ferris ME, Gipson DS, Kimmel PL, Eggers PW (2006) Trends in treatment and outcomes of survival of adolescents initiating end-stage renal disease care in the United States of America. Pediatr Nephrol 21:1020–1026
Goldstein SL, Graham N, Burwinkle T, Warady B, Farrah R, Varni JW (2006) Health-related quality of life in pediatric patients with ESRD. Pediatr Nephrol 21:846–850
Goldstein SL, Graham N, Warady BA, Seikaly M, McDonald R, Burwinkle TM, Limbers CA, Varni JW (2008) Measuring health-related quality of life in children with ESRD: performance of the generic and ESRD-specific instrument of the Pediatric Quality of Life Inventory (PedsQL). Am J Kidney Dis 51:285–297
Goldstein SL, Rosburg NM, Warady BA, Seikaly M, McDonald R, Limbers C, Varni JW (2009) Pediatric end stage renal disease health-related quality of life differs by modality: a PedsQL ESRD analysis. Pediatr Nephrol 24:1553–1560
McKenna AM, Keating LE, Vigneux A, Stevens S, Williams A, Geary DF (2006) Quality of life in children with chronic kidney disease-patient and caregiver assessments. Nephrol Dial Transplant 21:1899–1905
Riano-Galan I, Malaga S, Rajmil L, Ariceta G, Navarro M, Loris C, Vallo A (2009) Quality of life of adolescents with end-stage renal disease and kidney transplant. Pediatr Nephrol 24:1561–1568
Gerson AC, Wentz A, Abraham AG, Mendley SR, Hooper SR, Butler RW, Gipson DS, Lande MB, Shinnar S, Moxey-Mims MM, Warady BA, Furth SL (2010) Health-related quality of life of children with mild to moderate chronic kidney disease. Pediatrics 125:e349–e357
Irwin DE, Stucky BD, Thissen D, Dewitt EM, Lai JS, Yeatts K, Varni JW, DeWalt DA (2010) Sampling plan and patient characteristics of the PROMIS pediatrics large-scale survey. Qual Life Res 19:585–594
Yeatts KB, Stucky B, Thissen D, Irwin D, Varni JW, DeWitt EM, Lai JS, DeWalt DA (2010) Construction of the Pediatric Asthma Impact Scale (PAIS) for the Patient-Reported Outcomes Measurement Information System (PROMIS). J Asthma 47:295–302
Varni JW, Stucky BD, Thissen D, Dewitt EM, Irwin DE, Lai JS, Yeatts K, Dewalt DA (2010) PROMIS Pediatric Pain Interference Scale: an item response theory analysis of the pediatric pain item bank. J Pain 11:1109–1119
Irwin DE, Stucky BD, Langer MM, Thissen D, Dewitt EM, Lai JS, Yeatts KB, Varni JW, Dewalt DA (2011) PROMIS Pediatric Anger Scale: an item response theory analysis. Qual Life Res 21:697–706
DeWitt EM, Stucky BD, Thissen D, Irwin DE, Langer M, Varni JW, Lai JS, Yeatts KB, Dewalt DA (2011) Construction of the eight-item patient-reported outcomes measurement information system pediatric physical function scales: built using item response theory. J Clin Epidemiol 64:794–804
Varni JW, Seid M, Kurtin PS (2001) PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care 39:800–812
Irwin DE, Stucky B, Langer MM, Thissen D, Dewitt EM, Lai JS, Varni JW, Yeatts K, DeWalt DA (2010) An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales. Qual Life Res 19:595–607
Selewski DT, Collier DN, MacHardy J, Gross HE, Pickens EM, Cooper AW, Bullock S, Earls MF, Pratt KJ, Scanlon K, McNeill JD, Messer KL, Lu Y, Thissen D, DeWalt DA, Gipson DS (2013) Promising insights into the health related quality of life for children with severe obesity. Health Qual Life Outcomes 11:29
Gipson DS, Selewski DT, Massengill SF, Wickman L, Messer KL, Herreshoff E, Bowers C, Ferris ME, Mahan JD, Greenbaum LA, MacHardy J, Kapur G, Chand DH, Goebel J, Barletta GM, Geary D, Kershaw DB, Pan CG, Gbadegesin R, Hidalgo G, Lane JC, Leiser JD, Plattner BW, Song PX, Thissen D, Liu Y, Gross HE, DeWalt DA (2013) Gaining the PROMIS perspective from children with nephrotic syndrome: a Midwest pediatric nephrology consortium study. Health Qual Life Outcomes 11:30
Hinds PS, Nuss SL, Ruccione KS, Withycombe JS, Jacobs S, DeLuca H, Faulkner C, Liu Y, Cheng YI, Gross HE, Wang J, DeWalt DA (2013) PROMIS pediatric measures in pediatric oncology: valid and clinically feasible indicators of patient-reported outcomes. Pediatr Blood Cancer 60:402–408
U.S. Department of Health and Human Services, Food and Drug Administration. Guidance for industry: Patient-reported outcomes measures. Avaliable at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf
Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A (1976) A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58:259–263
Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G (2003) National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 139:137–147
Cohen J (ed) (1988) Statistical power analysis for the behavioral sciences. Lawrence Earlbaum Associates, Hillsdale, NJ
Gipson DS, Trachtman H, Kaskel FJ, Radeva MK, Gassman J, Greene TH, Moxey-Mims MM, Hogg RJ, Watkins SL, Fine RN, Middleton JP, Vehaskari VM, Hogan SL, Vento S, Flynn PA, Powell LM, McMahan JL, Siegel N, Friedman AL (2011) Clinical trials treating focal segmental glomerulosclerosis should measure patient quality of life. Kidney Int 79:678–685
Gledhill J, Rangel L, Garralda E (2000) Surviving chronic physical illness: psychosocial outcome in adult life. Arch Dis Child 83:104–110
Morton MJ, Reynolds JM, Garralda ME, Postlethwaite RJ, Goh D (1994) Psychiatric adjustment in end-stage renal disease: a follow up study of former paediatric patients. J Psychosom Res 38:293–303
Fadrowski J, Cole SR, Hwang W, Fiorenza J, Weiss RA, Gerson A, Furth SL (2006) Changes in physical and psychosocial functioning among adolescents with chronic kidney disease. Pediatr Nephrol 21:394–399
Gerson A, Hwang W, Fiorenza J, Barth K, Kaskel F, Weiss L, Zelikovsky N, Fivush B, Furth S (2004) Anemia and health-related quality of life in adolescents with chronic kidney disease. Am J Kidney Dis 44:1017–1023
Gerson AC, Riley A, Fivush BA, Pham N, Fiorenza J, Robertson J, Chandra M, Trachtman H, Weiss R, Furth SL (2005) Assessing health status and health care utilization in adolescents with chronic kidney disease. J Am Soc Nephrol 16:1427–1432
Ruth EM, Landolt MA, Neuhaus TJ, Kemper MJ (2004) Health-related quality of life and psychosocial adjustment in steroid-sensitive nephrotic syndrome. J Pediatr 145:778–783
Park KS, Hwang YJ, Cho MH, Ko CW, Ha IS, Kang HG, Cheong HI, Park YS, Lee YJ, Lee JH, Cho HY (2012) Quality of life in children with end-stage renal disease based on a PedsQL ESRD module. Pediatr Nephrol 27:2293–2300
Acknowledgements
The investigators are indebted to the children and families who graciously participated in this study.
The Patient-Reported Outcomes Measurement Information System (PROMIS) is an NIH Roadmap initiative to develop a computerized system measuring PROs in respondents with a wide range of chronic diseases and demographic characteristics.
PROMIS II was funded by cooperative agreements with a Statistical Center (Northwestern University, PI: David Cella, Ph.D., 1U54AR057951), a Technology Center (Northwestern University, PI: Richard C. Gershon, Ph.D., 1U54AR057943), a Network Center (American Institutes for Research, PI: Susan (San) D. Keller, Ph.D., 1U54AR057926) and 13 Primary Research Sites, which may include more than one institution (State University of New York, Stony Brook, PIs: Joan E. Broderick, Ph.D. and Arthur A. Stone, Ph.D., 1U01AR057948; University of Washington, Seattle, PIs: Heidi M. Crane, M.D., M.P.H., Paul K. Crane, M.D., M.P.H., and Donald L. Patrick, Ph.D., 1U01AR057954; University of Washington, Seattle, PIs: Dagmar Amtmann, Ph.D. and Karon Cook, Ph.D., 1U01AR052171; University of North Carolina, Chapel Hill, PI: Darren A. DeWalt, M.D., M.P.H., 2U01AR052181; Children’s Hospital of Philadelphia, PI: Christopher B. Forrest, M.D., Ph.D., 1U01AR057956; Stanford University, PI: James F. Fries, M.D., 2U01AR052158; Boston University, PIs: Stephen M. Haley, Ph.D. and David Scott Tulsky, Ph.D. (University of Michigan, Ann Arbor), 1U01AR057929; University of California, Los Angeles, PIs: Dinesh Khanna, M.D. and Brennan Spiegel, M.D., M.S.H.S., 1U01AR057936; University of Pittsburgh, PI: Paul A. Pilkonis, Ph.D., 2U01AR052155; Georgetown University, PIs: Carol. M. Moinpour, Ph.D. (Fred Hutchinson Cancer Research Center, Seattle) and Arnold L. Potosky, Ph.D., U01AR057971; Children’s Hospital Medical Center, Cincinnati, PI: Esi M. Morgan DeWitt, M.D., M.S.C.E., 1U01AR057940; University of Maryland, Baltimore, PI: Lisa M. Shulman, M.D., 1U01AR057967; and Duke University, PI: Kevin P. Weinfurt, PhD, 2U01AR052186). NIH Science Officers on this project have included Deborah Ader, Ph.D., Vanessa Ameen, M.D., Susan Czajkowski, Ph.D., Basil Eldadah, M.D., Ph.D., Lawrence Fine, M.D., Dr.P.H., Lawrence Fox, M.D., Ph.D., Lynne Haverkos, M.D., M.P.H., Thomas Hilton, Ph.D., Laura Lee Johnson, Ph.D., Michael Kozak, Ph.D., Peter Lyster, Ph.D., Donald Mattison, M.D., Claudia Moy, Ph.D., Louis Quatrano, Ph.D., Bryce Reeve, Ph.D., William Riley, Ph.D., Ashley Wilder Smith, Ph.D., M.P.H., Susana Serrate-Sztein, M.D., Ellen Werner, Ph.D. and James Witter, M.D., Ph.D. This manuscript was reviewed by PROMIS reviewers before submission for external peer review.
Conflicts of interest
The authors have no financial relationships or conflicts of interest relevant to this article to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Selewski, D.T., Massengill, S.F., Troost, J.P. et al. Gaining the Patient Reported Outcomes Measurement Information System (PROMIS) perspective in chronic kidney disease: a Midwest Pediatric Nephrology Consortium study. Pediatr Nephrol 29, 2347–2356 (2014). https://doi.org/10.1007/s00467-014-2858-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-014-2858-8