Abstract
Reliable reference values of enzymatically assayed serum creatinine categorized in small age intervals are lacking in young children. The aim of this study was to determine reference values for serum creatinine during the first year of life and study the influence of gender, weight and height on these values. Serum creatinine determinations between 2003 and 2008 were retrieved from the hospital database. Strict exclusion criteria ensured the selection of patients without kidney damage. Correlation analysis was performed to evaluate the relation between height, weight and serum creatinine; the Mann–Whitney test was used to evaluate the relation between gender and serum creatinine. A broken stick model was designed to predict normal serum creatinine values. Mean serum creatinine values were found to decrease rapidly from 55 μmol/L on day 1 to 22 μmol/L in the second month of life; they then stabilized at 20 μmol/L until the seventh month, followed by a slight increase. No significant relation was found between serum creatinine and gender, weight and height. We present here reference values of serum creatinine in infants not at risk of decreased renal function. The absence of a relationship with gender, weight and height confirms that height-based equations to estimate glomerular filtration rate are less useful in patients of this age group.
Similar content being viewed by others
Introduction
Screening for renal disease or monitoring of renal function should be based on an adequate estimation of the glomerular filtration rate (GFR). The most commonly used marker to estimate GFR is serum creatinine. Creatinine is a break-down product of creatine phosphate in muscle and is freely filtered by the glomerulus. Although estimation of the GFR using creatinine is not as specific and sensitive as, for example, an inulin clearance assay, the method is inexpensive, fast, and minimally invasive. The Jaffé reaction, based on a colorimetric method first described by Jaffé in 1886 [1], has long been the cornerstone of routine laboratory assays of creatinine. The major disadvantage of this method is its lack of accuracy caused by, among other factors, interference from conditions such as hyperbilirubinaemia, hypertriglyceridaemia, haemolysis as well as from proteins and ketone bodies [2]. In recent years, enzymatic methods that have less interference with other substances have widely replaced the Jaffé method [3].
Several studies have been performed to develop equations for calculating the GFR using serum creatinine and patient characteristics, such as age, body height and weight [4–8]. Most of the equations in children are based on serum creatinine levels determined by the Jaffé method [9], and many of these have been established based on results obtained in small cohorts; consequently, they may lack precision [10]. An additional complicating factor is that in young children the use of a GFR formula is made more difficult by the rapid increase in GFR during the first year of life—approximately 20 mL/min per 1.73 m2 at birth to 80–120 mL/min per 1.73 m2 at 12 months [11]. It would seem, therefore, that sensitive, age-corrected reference values of serum creatinine are required that refer to small age intervals.
Available studies on reference values of serum creatinine in children less than 1 year of age are given in Table 1. Only a few of these studies are based on the standard enzymatic method currently in widespread use. As these types of studies are usually performed in hospital populations, patient selection must be very strict to prevent the inclusion of patients with kidney damage or an otherwise abnormal serum creatinine; patients with high levels of substances known to interfere with creatinine assessment must also be excluded [3]. The exclusion criteria in the studies listed in Table 1 do not appear to have been strict enough. We therefore designed the retrospective study reported here to determine new reference values of serum creatinine assayed enzymatically in infants up to 12 months of age. By using a large, carefully selected cohort of patients, our aim was to improve currently used reference values. We compared these new reference values to those of other studies and also evaluated the influence of gender, weight and height on the serum creatinine concentration.
Patients and methods
All serum creatinine values determined in children less than 1 year of age in the Erasmus MC–Sophia Children’s Hospital between January 2003 and December 2008 were extracted from the data base. Blood sampling had been done by capillary or venous puncture. Creatinine had been determined by an enzymatic assay (Creatinine Plus; Roche Diagnostics, Branchburg, NJ) on an Hitachi 912 analyzer, as described elsewhere [12]. During the period of sample collection, the interassay coefficient of variation (CV) was less than 2%. When performed in the SKML (Dutch foundation for Quality Assurance in Clinical Diagnostic Laboratories), our creatinine assay, calibrated to the high-performance liquid chromatography method, had a bias of 0.65%.
Exclusion criteria
In order to include measurements of children with normal serum creatinine level only, several steps of exclusion were carried out. Firstly, to prevent the influence of serial measurements, we randomly selected one assay per patient. Secondly, all determinations requested by the departments of Paediatric Nephrology, Paediatric Oncology and the Neonatal and Paediatric Intensive Care Units were excluded. Thirdly, the remaining patients were screened for pathophysiological conditions that could cause renal damage (Table 2).
Patients born preterm (<37 weeks of gestational age) or small for gestational age (birth weight <10th percentile according to The Netherlands Perinatal Registry 2007 [13]) were excluded because of their expected immature renal function. All patients with a proven or suspected syndrome were also excluded, for many syndromes are associated with multiple organ anomalies, including renal pathology. Finally, during measurement on the Hitachi 912, those indices possibly associated with interference with the enzymatic method (hyperbilirubinaemia, hypertriglyceridaemia and haemolysis) were measured in each sample. Samples with indications of haemolysis, hyperbilirubinaemia or hypertriglyceridaemia were excluded. All patients with muscle disease were also excluded.
All assay results from 1- to 7-day-old children were included in view of the expected rapid initial decline in serum creatinine during the first days of life. For older children up to 3 months of age, 15 random determinations per week were included; for those between 3 and 12 months, five random determinations per week.
The exclusion procedure was carried out by two physicians who were blinded to the serum creatinine levels.
Statistical analysis
Visual inspection of scatter plots of serum creatinine versus age did not reveal a simple functional relationship. Prior knowledge suggested a rapid initial decrease, followed by a plateau and then a gradual rise. This pattern remained after logarithmic transformation of both variables.
Therefore, a “broken stick” relationship with three log-linear segments was fitted. These segments were tied together at two breakpoints. The positions of the breakpoints (defined as K1 and K2) on the x-axis were estimated using non-linear regression analysis. The initial slope before the first breakpoint and the slope after the second breakpoint were also calculated.
The relationship can be written as:
where X denotes 2log(age in years), Y denotes the 10log[creat (μmol/L)], K1 is the breakpoint between the initial decrease and the plateau and K2 is the breakpoint between the plateau and the following increase. A denotes the intercept, B is the initial slope before breakpoint K1 and C is the second slope after breakpoint K2.
Using this approach, we first determined the parameters in the relationship and then estimated the standard deviation of the residuals (=difference between predicted value by the model and the real value measured), which allows us to calculate the 2.5th and 97.5th percentiles. We then calculated our model and its limits and anti-logged the values, which gave us the median and 2.5th and 97.5th percentiles.
To check the relationship between the residuals of this model and the children’s height and weight, we used Spearman’s correlation analysis. The influence of gender was estimated with the Mann–Whitney test. A difference was assessed as significant at P < 0.05. The software used was Statistical Package for the Social Sciences (SPSS) ver. 16 (SPSS, Chicago, IL).
After having determined the ‘new’ reference values, we compared these with those from other studies. A valid comparison with the results of Pottel et al. [14] necessitated adjustment of our data to the method these authors reported as they only excluded patients with serial measurements and outlying values.
Results
Between January 2003 and December 2008 a total of 29,839 determinations of serum creatinine were performed in 5,608 patients younger than 1 year of age. Values from patients who did not meet the inclusion criteria were excluded, resulting in a total of 12,205 values. Random selection of one assay per patient resulted in 3575 assays for study.
A total of 748 patients were excluded from the study for the reasons given in our exclusion criteria. Table 2 shows the number of exclusions for each criterion. Some patients were excluded by more than one criterion. A total of 442 patients was included randomly; 67 during the first week, categorized to days of life; 15 during each week in the next 12 weeks of life; five during each week of the remaining weeks up to the first year of life. There was a male preponderance among these patients (57.7%). Body weight and height at time of determination was available in 397 (89.8%) and 176 (39.8%) of these patients, respectively.
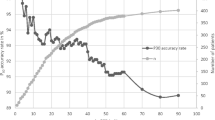
The individual creatinine values of the included patients and the graph of the model are shown in Fig. 1. The reference values for the separate time intervals are shown in Table 3. With this model, the R 2 is 0.63. The intercept was estimated as 1.75. The first breakpoint is on day 65 and the second breakpoint on day 216. The initial slope is −0.07, which corresponds to a 15% decrease in serum creatinine with doubling of the age before day 65. The second slope is 0.045, which corresponds to an 11% increase in serum creatinine with doubling of the age after day 216. The geometric mean at plateau phase was 20 μmol/L.
The standard deviation of the residuals of the model was 0.084. The 2.5th and 97.5th percentiles are given by the formula: 10predicted values ± 2 × 0.084, respectively.
The correlation analysis showed that there was no correlation between the residuals and length and weight of the patient (r = −0.016, P = 0.75 and r = −0.058, P = 0.45, respectively). Therefore, following adjustment for age, both variables did not significantly influence creatinine values. The Mann–Whitney test comparing the residuals between boys and girls also did not show a significant effect of gender (P = 0.11).
A comparison of our results with those of Pottel et al. [14], the study most similar to ours, revealed a clear difference (Fig. 2). Using the selection procedure applied in that study virtually extended our data set to 2243 patients and increased the mean values by 20%, thus corresponding to the data of Pottel et al.
Comparison between the results of Pottel et al. [14] (interupted lines) and our results (continuous lines). Continuous lines 97.5th percentile (upper), median (middle) and the 2.5th percentile (lower) of our data. Interrupted lines 97.5th percentile (upper), median (middle) and the 2.5th percentile (lower) of the data of Pottel et al.
Discussion
Insight into the GFR of children of all age groups enables clinicians to adjust the dosage of drugs eliminated by the kidney and thereby avoid the use of nephrotoxic drugs. Estimation of GFR by formulas based on body size is not applicable to infants, as we confirmed with our data. Furthermore, serum creatinine values considerably change over time. Reliable reference values of serum creatinine in infants are scarce for several reasons. First, in many studies, they have been derived with the flawed Jaffe method, not the enzymatic assay. Second, the numbers of infants included in such studies have tended to be small and the selection of patients not optimal. Third, the age intervals used are too wide to accurately reflect the rapid changes in creatinine during the first weeks of life. Consequently, in our retrospective study, we collected values of serum creatinine in children younger than one year of age from a large population, carefully selected for normal kidney function and divided over adequate intervals. Careful selection is a must because this type of study can only be performed in hospital populations: it is considered unethical to draw blood from healthy infants. Even the slightest suspicion of dysplasia or dysfunction of the kidneys should be reason for exclusion. The long list of exclusion criterions we used was aimed at selecting a population with normal kidney function.
The results of our study confirm the well-known rapid decline of serum creatinine during the first weeks of life (Fig. 1). At birth, serum creatinine reflects the relatively high maternal level of serum creatinine, which is about 65–70 μmol/L for women aged 20–40 years [14]. Pottel et al. reported a rise on day 1 after birth [14]. We did not find this, perhaps due to the absence of cord blood samples and the low number of measurements on day 1. A temporary increase may be a physiological phenomenon, reflecting the sudden vanishing of the high maternal clearance, some production of creatinine by the child, in combination with a low renal clearance by the child. However, it may also be due to the inclusion of patients with asphyxia or premature birth. The pace at which the serum creatinine level decreases in the weeks after birth gradually slows down. A plateau is reached between approximately days 65 and 220 as a result of two physiological developments: (1) ongoing kidney maturation and, consequently, increased filtration of creatinine; (2) a simultaneous increase in absolute creatinine level due to increasing muscle mass. Thereafter, the level gradually rises again, caused by increasing muscle mass; the kidney is now almost mature, and the GFR relative to body surface area will not increase much further. This complicated pattern of changing levels of serum creatinine is a major reason that small intervals for the reference values need to be defined, especially during the first few weeks of life.
Visual inspection of the data points in relation to the fitted model reveals that our broken-stick model fits the data quite well. This was confirmed with the R2, which was 0.63. When data on the first few days of life were left out of the analysis—on which there were relatively few inclusions—our reference values remained unaltered on visual inspection.
Our data are in agreement with those of Schlebusch et al. [15] in the age group between 2 months and 1 year of age. These researchers also used the enzymatic determination method of creatinine. However, they found a somewhat wider range of values in this period, namely 19, 14 and 35 μmol/L for the mean and 2.5th and 97.5th percentiles, respectively, versus 20, 14 and 30 μmol/L in the present study. Comparison of the data in the first weeks of life is not possible because Schlebusch et al. used larger age intervals.
Ceriotti et al. [16] set out to model age-adjusted paediatric reference intervals using the original data presented by Schlebusch et al. Following the approach of Royston and Wright, they found a wider range of reference values. For example, the 2.5th and 97.5th percentiles at day 7 were 22 and 73 μmol/L, respectively, compared to 23–51 μmol/L in our study. The wider ranges reported by Schlebusch et al. [15] and Ceriotti et al. [16] may be explained by the smaller number of patients included—41 versus 264 in our study.
Our reference values in the first weeks of life are lower than those reported by Pottel et al. [14] (Fig. 2). This is probably due to the different exclusion criteria applied. Pottel et al. only excluded serial measurements and outliers, whereas we excluded all patients at risk for increased creatinine, including prematurely born patients. The latter have higher values of serum creatinine due to immature renal function [17–19]. Using the selection method applied by Pottel et al. on our database resulted in similar curves. We suggest that the higher values found in the study by Pottel et al. may be caused by the inclusion of patients with a higher creatinine level than that found in the normal term population, leading to overestimation of the reference values of serum creatinine.
In our young population, patients’ gender, height and weight did not influence the serum creatinine concentration. The absent influence of gender has been confirmed in other studies for this age group [14, 20]. The finding that height and weight are not related to the level of serum creatinine emphasizes that one should be careful using formulas that calculate the GFR from serum creatinine and height in children of this age group.
Although the reference values of serum creatinine presented in this study may help clinicians to assess renal function, a marker of renal function that is stable over time and not influenced by muscle mass, tubular secretion and other factors would be preferable. Promising markers may be Cystatin C and beta-trace protein [21–23], of which the latter is still under evaluation. In contrast to serum creatinine, serum Cystatin C concentration is not influenced by age, weight, height, gender and body composition in older patients [24, 25]. The use of such a stable marker should simplify the interpretation of test results.
In conclusion, through the use of a carefully selected sample of patients less than 1 year of age from a large hospital population, we have been able to present reliable reference values for enzymatically assayed serum creatinine in children of this age group.
References
Jaffé M (1886) Über den Niederschlag welchen Pikrinsaure in normalen Harn erzeugt und über eine neue Reaktion des Kreatinines. Z Physiol Chem 10:391–400
Spencer K (1986) Analytical reviews in clinical biochemistry: the estimation of creatinine. Ann Clin Biochem 23:1–25
Cobbaert CM, Baadenhuijsen H, Weykamp CW (2009) Prime time for enzymatic creatinine methods in pediatrics. Clin Chem 55:549–558
Schwartz GJ, Brion LP, Spitzer A (1987) The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 34:571–590
Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A (1976) A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58:259–263
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:461–470
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
van Rossum LK, Mathot RA, Cransberg K, Zietse R, Vulto AG (2005) Estimation of the glomerular filtration rate in children: which algorithm should be used? Pediatr Nephrol 20:1769–1775
International Federation of Clinical Chemistry and Laboratory Medicine, Working Group on Standardization of Glomerular Filtration Rate Assessment, Panteghini M, Myers GL, Miller WG, Greenberg N (2006) The importance of metrological traceability on the validity of creatinine measurement as an index of renal function. Clin Chem Lab Med 44:1287–1292
Gretz N, Schock D, Sadick M, Pill J (2007) Bias and precision of estimated glomerular filtration rate in children. Pediatr Nephrol 22:167–169
Wahl EF, Lahdes-Vasama TT, Churchill BM (2003) Estimation of glomerular filtration rate and bladder capacity: the effect of maturation, ageing, gender and size. Br J Urol Int 91:255–262
Junge W, Wilke B, Halabi A, Klein G (2004) Determination of reference intervals for serum creatinine, creatinine excretion and creatinine clearance with an enzymatic and a modified Jaffe method. Clin Chim Acta 344:137–148
Perinatreg (2007) Birth weight centiles and SD. Available at www.perinatreg.nl. Accessed 7 May 2009
Pottel H, Vrydags N, Mahieu B, Vandewynckele E, Croes K, Martens F (2008) Establishing age/sex related serum creatinine reference intervals from hospital laboratory data based on different statistical methods. Clin Chim Acta 396:49–55
Schlebusch H, Liappis N, Kalina E, Klein C (2002) High sensitive CRP and creatinine: reference intervals from infancy to childhood. J Lab Med 26:341–346
Ceriotti F, Boyd JC, Klein G, Henny J, Queralto J, Kairisto V, Panteghini M (2008) Reference intervals for serum creatinine concentrations: assessment of available data for global application. Clin Chem 54:559–566
Bueva A, Guignard JP (1994) Renal function in preterm neonates. Pediatr Res 36:572–577
Savory DJ (1990) Reference ranges for serum creatinine in infants, children and adolescents. Ann Clin Biochem 27:99–101
van den Anker JN, de Groot R, Broerse HM, Sauer PJ, van der Heijden BJ, Hop WC, Lindemans J (1995) Assessment of glomerular filtration rate in preterm infants by serum creatinine: comparison with inulin clearance. Pediatrics 96:1156–1158
Gomez P, Coca C, Vargas C, Acebillo J, Martinez A (1984) Normal reference-intervals for 20 biochemical variables in healthy infants, children, and adolescents. Clin Chem 30:407–412
Pucci L, Triscornia S, Lucchesi D, Fotino C, Pellegrini G, Pardini E, Miccoli R, Del Prato S, Penno G (2007) Cystatin C and estimates of renal function: searching for a better measure of kidney function in diabetic patients. Clin Chem 53:480–488
Hojs R, Bevc S, Ekart R, Gorenjak M, Puklavec L (2008) Serum cystatin C-based equation compared to serum creatinine-based equations for estimation of glomerular filtration rate in patients with chronic kidney disease. Clin Nephrol 70:10–17
White CA, Akbari A, Doucette S, Fergusson D, Hussain N, Dinh L, Filler G, Lepage N, Knoll GA (2009) Estimating GFR using serum beta trace protein: accuracy and validation in kidney transplant and pediatric populations. Kidney Int 76:784–791
Bokenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J (1998) Cystatin C-a new marker of glomerular filtration rate in children independent of age and height. Pediatrics 101:875–881
Bokenkamp A, Domanetzki M, Zinck R, Schumann G, Brodehl J (1998) Reference values for cystatin C serum concentrations in children. Pediatr Nephrol 12:125–129
Jury DR (1979) Serum creatinine concentration in children: normal values for sex and age. N Z Med J 90:453–456
Feldman H, Guignard JP (1982) Plasma creatinine in the first month of life. Arch Dis Child 57:123–126
Trompeter RS, Al-Dahhan J, Haycock GB, Chik G, Chantler C (1983) Normal values for plasma creatinine concentration related to maturity in normal term and preterm infants. Int J Pediatr Nephrol 4:145–148
Gordjani N, Burghard R, Leititis JU, Brandis M (1988) Serum creatinine and creatinine clearance in healthy neonates and prematures during the first 10 days of life. Eur J Pediatr 148:143–145
Sugita O, Uchiyama K, Yamada T, Sato T, Okada M, Takeuchi K (1992) Reference values of serum and urine creatinine, and of creatinine clearance by a new enzymatic method. Ann Clin Biochem 29:523–528
Greeley C, Snell J, Colaco A, Beatey J, Bailey J, Bjorn S, Rifai N, Hicks J, Soldin S (1993) Pediatric reference ranges for electrolytes and creatinine. Clin Chem 39:1172
Soldin S, Hicks J, Bailey J, Cook J, Beatey J (1997) Pediatric reference ranges for creatinine on the Hitachi 747. Clin Chem 43:S198
Finney H, Newman DJ, Thakkar H, Fell JM, Price CP (2000) Reference ranges for plasma cystatin C and creatinine measurements in premature infants, neonates, and older children. Arch Dis Child 82:71–75
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Boer, D.P., de Rijke, Y.B., Hop, W.C. et al. Reference values for serum creatinine in children younger than 1 year of age. Pediatr Nephrol 25, 2107–2113 (2010). https://doi.org/10.1007/s00467-010-1533-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-010-1533-y