Background
We aimed to assess the outcomes including the effect on quality of life (QoL) of a group of patients having a minimally invasive esophagectomy (MIE).
Methods
Patients with esophageal cancer were offered MIE over a 22-month period. Data on outcomes were collected prospectively, including formal quality-of-assessments.
Results
There were 25 patients offered MIE. Two patients were converted to a laparotomy to improve the lymphadenectomy. There were no deaths. Respiratory problems (pneumonia, 28%) were the most common in the 64% of patients who had a complication. The median blood loss was 300 ml, time of surgery 330 min, and time to discharge 11 days. There was a decrease in the measured QoL both in general and specifically for the esophageal patients, taking 18–24 months to return to baseline.
Conclusions
MIE was performed with morbidity similar to other approaches. There were no clear benefits shown in this group of patients with respect to postoperative recovery or short- to medium-term QoL.
Similar content being viewed by others
Unfortunately, the majority of patients undergoing esophagectomy will die of their disease, with most series suggesting that 40% will die in the first year after the operation [12]. Thus the issues of early recovery and quality of life after surgery, notably in the medium term, are of great importance when, for many patients, the operation is palliative. Previous attempts at reducing the degree of surgical trauma in open surgery by avoiding a thoracotomy, using blunt dissection in the mediastinum, have not shown a major benefit in comparative and randomised trials [16].
Minimally invasive techniques and approaches have been developed with the hope that these will have a beneficial impact on early complication rates, return to normal activity, and medium- to long-term quality of life, while providing, at least, equal survival figures when compared with traditional open surgery. A variety of techniques including thoracoscopy [14, 17], laparoscopy [9], and hand-assisted techniques [18] have been reported. It also seems reasonable to assume that minimally invasive esophagectomy (MIE) would improve a patient’s short- to medium-term quality of life if the patient’s return to normal activity were hastened. If there were evidence for this effect, it would be a persuasive argument to consider offering this approach to our patients with esophageal cancer.
Our unit has previously reported the use of thoracoscopic techniques to mobilize the esophagus [17]. We now wish to report our technique and results from a more complete MIE and reconstruction. We have examined the safety of the technique with medium-term follow up, describing early and late morbidity, as well as considering the effect on the quality of life of those patients undergoing this procedure.
Methods
Between December 1998 and October 2000, data were collected prospectively on a consecutive series of patients undergoing MIE and stored on an electronic database (Microsoft Access). Patients were selected for MIE if they were medically fit for surgery and had tumors confined to the esophagus or esophagogastric junction not extending more than 1 cm into the gastric cardia. Patients were assessed radiologically with CT scanning. Endoscopic ultrasound (EUS) was not available at our institution during the period of study. No patient was selected or excluded on the basis of either perceived technical difficulty of the operation or “favorable pathology.” Information relating to the operation included blood loss, operative time, and time in the intensive care ward. Operation times were measured for each component of the surgery and were recorded from commencement of the surgery but did not include anesthetic time prior to skin preparation.
Postsurgery data included length of hospital stay as well as morbidity. Any event that was considered to be outside a normal recovery was considered to be a complication. In particular, all respiratory problems were documented carefully. If antibiotics were considered necessary, this was documented as a significant infection. Patients have been followed every 3 months for 2 years, every 6 months for two years, and annually thereafter. Follow-up assessment included a history, clinical examination, and quality-of-life measurements. Further investigations were performed according to the clinical indications.
Quality-of-life (QoL) assessment: Patients completed QoL questionnaires prior to the commencement of treatment and then at 3-month intervals for the first 12 months and then every 6 months. The QoL questionnaires used included the EORTC QLQ-C30 and the EORTC QLQ-OES18, which has been validated as a tool specific for patients with esophageal cancer [4, 5]. Our unit had been given permission to use this tool prior to its published validation. From the QLQ-C30 questionnaires the focus was physical function, role function, emotional function, and global QOL. From the QLQ-OES18 the focus was dysphagia, deglutition (includes indigestion), eating. and cough.
Technique
The operation began with a thoracoscopic mobilization of the thoracic esophagus and then the laparoscopic gastric mobilization and cervical component were performed concurrently by two of the senior surgeons (M.S., D.G.). The technique for thoracoscopic dissection has been described previously [17].
After induction of general anesthesia with double-lumen endotracheal intubation, and placement of an epidural catheter and central venous catheter, the patient is placed prone on the operating table with both arms flexed and abducted on padded arm boards to displace the scapulae laterally. The video monitor is placed on the left side of the bed. Both surgeon and assistant stand beside the right chest of the patient, which is on the left-hand side of the operating table. The right chest is prepared and the right lung is deflated. A 12-mm blunt thoracic port (Ethicon-Endosurgery) is placed in the intercostal space distal to the tip of the scapula by blunt dissection, and a 30° endoscope is introduced via this port. A second 12-mm port is placed under vision medial to the scapula at a level just below the azygos vein. A third 12-mm port is placed just above the diaphragm. A fourth port (5-mm), placed between the superior and inferior ports just lateral to the paraspinal muscles, may be used to assist in dissection around the distal esophagus and to offer retraction for the dissection of the proximal esophagus. Using the hooked diathermy, electrocautery dissection begins with the pleura adjacent to the azygos vein, and the vein is divided using a 45-mm endoscopic stapler. The pleura are then divided longitudinally anterior and posterior to the esophagus from the azygos vein to the diaphragm, and a sling is passed around the esophagus and brought out alongside the camera port to provide retraction. Mobilization then continues distally to the hiatus, and proximally to the apex of the thorax. Small vessels are controlled with diathermy, while larger vessels are divided between endoscopic clips. The paraesophageal, subcarinal, and proximal bronchial lymph nodes are resected en-bloc with the esophagus, and the left and right main bronchi are carefully displayed. At the completion of the dissection, a single intercostal catheter is passed via the camera port and positioned in the apex of the thorax. The right lung is then reinflated under vision, the ports withdrawn, and the wounds sutured.
The patient is then repositioned supine on the table with the neck extended and the head turned to the right, allowing access to the lower left neck. The legs may be elevated in stirrups with the thighs flat, to allow the operating surgeon to stand between the legs and the camera assistant to stand on the patient’s left side. Alternatively, the patient may lie flat on the table with the surgeon standing on the patient’s left and the camera assistant on the patient’s right side.
The camera port is inserted into the abdomen in the midclavicular line at a site that is considered to offer access to the lower greater curve of the stomach and the hiatus of the diaphragm. Subsequent ports are inserted under vision. The usual positions are a 10-mm port placed in front of the tip of the ninth rib on the left; a 5-mm port placed at the level of the camera port in the midline, and in the left upper abdomen a port is placed around the mid-clavicular line on that side. A hole is made beside the xiphisternum to allow insertion of the Nathanson (Cook) liver retractor, which is positioned under vision to retract the liver and give access to the hiatus and the gastric fundus.
The greater curve of the stomach is defined and the gastro-epiploic arcade visualized. Using ultrasonic shears (Johnson and Johnson Medical), the lesser sac is entered distal to the vascular arcade. This allows better visualization of the arcade in an obese patient. The omental branches of the arcade are divided moving proximally, ensuring that the arcade remains intact. This dissection is taken up onto the fundus, where the short gastric vessels are divided to completely mobilize the fundus medially. The left crus of the diaphragm is defined, but the phreno-esophageal ligament is kept intact. The dissection is then moved to complete the mobilization of the greater curve, moving to the left to ensure that the arcade remains intact. The left side of the hiatus is then dissected, moving up from the gastro-hepatic ligament, which is divided. The posterior cardia region of the stomach is fully mobilized to define the left gastric pedicle. This is divided with a linear stapling device using vascular staples. The dissection is taken to the level of the hiatus. In the early part of the series, we mobilized the first and second parts of the duodenum completely using a further port placed in the patient’s right lower abdomen to allow better access to the duodenum. However, it became apparent that this step was not necessary; any adhesions to the gall bladder were divided, as we had no problems with the length of the gastric tube that we were able to construct. A pyloromyotomy is then performed using a combination of blunt and sharp dissection. If the mucosa is breached (as happened in two patients), then a pyloroplasty is performed endoscopically with a single-layer, full-thickness, continuous suture.
At this time the second surgeon commences the approach in the neck. An oblique incision is made along the anterior border of the lower third of the sternocleidomastoid muscle. The major vessels in the neck are retracted laterally and the thyroid medially, to allow access to the prevertebral region. The esophagus is identified and then dissected to allow a sling to be passed around it. No retractors are placed below the level of the thyroid, to avoid compression of the recurrent laryngeal nerve. The esophagus is now bluntly mobilized down to meet with the mediastinal dissection. The abdominal surgeon then divides the phreno-esophageal ligaments and completes the inferior mediastinal mobilization. This allows the esophagus to be delivered in the neck and it is divided attaching a tape that is pulled down into the abdomen as the esophagus is pulled down by the abdominal surgeon.
A 5–6-cm incision is made over the site of the duodenum and the esophagus is delivered. It is pulled through a glove with the middle finger removed. The glove is then inserted into the abdomen so that when the whole esophagus is withdrawn, the glove covers the site of the tumor and thus the wound is protected. The stomach is delivered through the wound. A gastric tube is constructed by division of the lesser curve vessels, allowing a minimum of 5 cm from the palpable edge of the tumor if there is any extension below the esophago-gastric junction. The fat and nodal tissue on the lesser curve are removed en-bloc with specimen. The stomach is divided with a linear stapler and the staple line is oversewn. The specimen is put aside to allow the operating surgeon to formally dissect the nodes for analysis after the operation is completed.
The tape brought from the neck incision is attached to the uppermost point of the gastric tube and the stomach is inserted back into the abdomen. Orientation of the gastric tube is confirmed and the tube is drawn up to the neck using a combination of pull from above and pushing at the level of the hiatus. Once the stomach has been delivered into the neck, the abdominal surgeon inspects the operative site for problems. If it is considered necessary, a feeding jejunostomy is inserted by finding the proximal jejunum and taking it to the surface through a 1–2-cm incision. The right upper abdomen wound is closed in layers.
The surgeon at the neck performs an end-to-side anastomosis between the esophagus and the posterior wall of the gastric tube using a single layer of interrupted absorbable sutures. After completion of the anastomosis, a nasogastric tube is inserted into the gastric pull-up and a Penrose drain is placed into the neck to drain the anastomosis. All patients are then transferred to the intensive care unit.
At the end of the operation the surgeon dissects the nodal tissue from the specimen, taking care not to divide individual nodes. These are placed into separate labeled containers. The groups are the upper gastric/celiac nodes, which includes the cardia nodes; the lower mediastinal nodes; and the subcarinal nodes.
Results
During the study period, 25 patients had an endoscopic esophagectomy. There were 22 men and three women. The median age was 61 years (range 38–77 years). The median weight of patients was 80.5 kg (range 50–126 kg), and median American Society of Anesthesiologists (ASA) grading was 2 (range 1–3). The tumors were located in the lower esophagus (19), at the esophago-gastric junction (4), and mid-esophagus (2). The histologic subtypes were invasive adenocarcinoma (17), squamous cell carcinoma (4), and high-grade dysplasia/adenocarcinoma in situ in Barrett’s esophagus (4). Preoperative neoadjuvant chemo-radiotherapy was given to eight patients (32%) with invasive carcinoma as part of a trial protocol. No patient had postoperative adjuvant therapy. Patients were followed for a median of 32 months (range 2–55 months).
All 25 patients had the thoracic esophageal mobilization completed thoracoscopically. The laparoscopic procedure was completed in 23 of the 25 patients. Two procedures were converted to laparotomy because of tumor-related factors. In one patient the abdominal lymphadenectomy was technically difficult due to adipose tissue obscuring the lymphatic pedicle around the left gastric artery and vein. The second patient had a large tumor within the hiatus with associated nodal disease. It was decided that continuing the laparoscopic approach would compromize the lymphadenectomy and thus the dissection was completed via laparotomy. The results presented will be for all 25 patients.
The perioperative details are listed in Table 1. The total median operative time was 330 min, with a median blood loss of 300 ml, with only three patients requiring a transfusion. The median length of stay in the ICU was 19 h (range 13–312 h). Epidural catheters were placed in 24 patients preoperatively (one patient refused), and these remained postoperatively for a median of 4 days (range 0–5 days). Median length of stay was 11 days (range 7–49 days). When perioperative variables were compared for early stage (0, I, IIA) and late stage (IIB, III), there was no difference in the operative outcome parameters. We also saw no difference in the outcomes and complications when comparing the first 12 patients with the remaining 13 patients.
Complications occurred in 64% of patients. Most were of a minor nature not affecting the patient’s recovery. Respiratory complications occurred in 15 patients, with pneumonia in seven (28%), major atelectasis on chest radiograph in five (20%), pleural effusion in one (4%), and a residual pneumothorax in two (8%). Cardiac complications consisting of supraventricular arrhythmias occurred in seven patients. A single patient developed a chyle leak requiring a thoracoscopy and application of a clip to close the divided thoracic duct. There were two anastomotic leaks, presenting as a salivary discharge in the neck, which healed spontaneously. One patient suffered a small laceration to the posterior wall of the trachea 1 cm above the carina that was recognized intraoperatively and repaired by primary suture with no further consequences. One patient developed an early acute diaphragmatic herniation of small bowel into the right hemithorax, requiring operative correction via laparotomy. The most serious complication was a gastric tube necrosis requiring resection of the tube and formation of a temporary cervical esophagostomy. The cause of the necrosis was not obvious at the time of the second operation. No patient had vocal cord palsy. There were no deaths within 30 days of surgery; however, one patient had rapidly progressive disease diagnosed after discharge and died on day 46 from the metastases.
When examining the lymph node dissection, the number of nodes found in each region is shown in Table 1. The median number of nodes removed was 17.5. The tumors were T0 (4), T1 (4), T2 (2), T3 (13), and T4 (2) tumors. Lymph node involvement was found in 15 specimens (60%). The AJCC [1] stage of disease was stage 0 (4), stage I (4), stage IIA (2), stage IIB (4), and stage III [11]. No patient had esophageal or gastric margin involvement, but four (16%) had microscopic disease at the lateral margin.
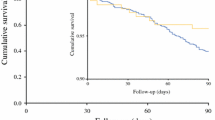
Fifteen patients (60%) have developed recurrence of their cancer since the surgery. The median time to recurrence was 11 months (mean 13 months, range 1–38 months). The site of first recurrence was distant in 11 patients, locoregional in two, and both distant and locoregional in two patients. The overall median survival was 32 months (range = 1–46 months). The survival curves for this group of patients are shown in Fig. 1.
From a functional point of view, 14 patients complained of significant gastroesophageal reflux, 12 being on long-term acid-suppressing medication. An endoscopy with anastomotic dilatation was required in nine patients for a median of two occasions (range 1–6), and two patients had symptoms of dumping syndrome that lasted four and 12 months, respectively.
Quality-of-life follow-up: Information was collected up to 36 months on all surviving patients. There were 19 patients alive at 12 months, 11 patients alive at 24 months, and 5 alive at 36 months. Based on the EORTC QLQ-C30 questionnaire, patients had a significant decrease in physical function, role function, and global health score immediately following surgery, and while there was a slow progressive improvement in all of these in the surviving patients, it took 18–24 months to approach baseline figures. Only global health status improved above the baseline level after 18 months (Fig. 2A) Using the EORTC QLQ-OES18 questionnaire, dysphagia, deglutition, eating problems, GI symptoms, and cough were assessed (Figs. 2B and 2C). Again, deglutition and eating problems took 18–24 months to return to baseline, while dysphagia deteriorated early postoperatively, but returned to baseline after between 6 and 9 months, and continued to improve to 18 months in this group of patients. Interestingly, troublesome cough is a common side effect among this group of patients, with early postoperative cough developing and worsening for 9 months, before improving, but not back to baseline by 24 months.
Discussion
To date few series have been published that demonstrate a clear benefit for using different components of MIE to resect the esophagus in patients with cancer. Previous reports from large-volume units staffed by experienced surgeons have shown that performing the thoracic component for esophagectomy using MIE can be done safely, with results at least comparable to open surgery [6, 11, 18]. The role of MIE for esophageal cancer has been questioned by some authors [14], but our own series of patients, who had a thoracoscopic approach to the esophageal mobilization, did not show any apparent compromise [17]. The laparoscopic approach to gastric mobilization has been reported to be safe and can be used in patients with impaired pulmonary function with reduced postoperative pain and improved early pulmonary function compared with laparotomy. Despite the advantages, this group reported the procedure to be time-consuming (mean operating time 511 min) and exposed patients to hypothermia (mean body temperature 34.3°C), resulting in delayed extubation [9].
DePaula et al. [6] and Swanstrom and Hansen [18] were the first to describe a series of total laparoscopic esophagectomies. The perceived benefits reported by Swanstrom and Hansen included decreased operative morbidity, decreased pain, shorter hospital stay, and earlier return to normal activities [18]. Nguyen and colleagues [13] reported advantages to adding thoracoscopy to the laparoscopic transhiatal approach, including improved visualization of the periesophageal structures, and more radical resection of mediastinal lymph nodes than that obtained by the open blunt dissection. Our group had a large experience with laparoscopic surgery to the hiatal region. This group of patients represents our experience with total gastric mobilization and added to our experience of thoraoscopic mobilization of the esophagus.
The largest series of total MIE has shown low morbidity and mortality [10]. Our patients had MIE with a small right upper quadrant abdominal incision to create the gastric tube. The difference in the morbidity compared to other reports may be due to the definition of a “significant” complication. We have included all adverse outcomes in our assessment. We report a slightly longer hospital stay, but there was no evidence that this was related to the abdominal incision. Other features, such as blood loss, time for surgery, and mortality, were comparable with the other reports [6, 18, 19]. Our most serious problem was necrosis of the gastric pull-up in one patient. No specific technical cause was identified at the time of the subsequent thoracotomy and laparotomy.
Respiratory complications following esophagectomy are common, with the incidence reported to be between 20% and 35% [3, 7, 8]. This has been relatively constant despite an emphasis on improved analgesia, early extubation, and vigorous physiotherapy. Variations in reporting occur because of the differences in definitions used for the various pulmonary complications. In a prospective report of the outcomes of 1,777 patients following esophagectomy, Bailey et al. reported pneumonia in 21.4% [2]. When the transthoracic and transhiatal approaches to esophagectomy were compared in a large multi-institutional review of 945 patients, pneumonia was seen in 26% of patients after a transthoracic approach compared with 18% for transhiatal esophagectomy, which was significantly different [14]. The approach to resection made no difference. Our respiratory complications included pneumonia (28%), as defined by the use of antibiotics to treat patients with a fever and chest radiography changes, and major atelectasis (20%). Most of these patients were not significantly disadvantaged by these problems. In their first publication of 77 patients who had MIE, Luketich et al. reported atelectasis in five and pneumonia in six patients. The definition of these complications was not clear. The reason our respiratory complication rate is higher may be multifactorial, possibly relating to the incision on the abdominal wall as well as the definitions we used to report the event as a complication. Whether the method of access or the mediastinal dissection of the esophagus is the major contributor to the respiratory problems is not known. One suspects the latter may be more important, given the persistence of these problems despite minimally invasive approaches to resection and reconstruction being used.
We did not select patients based on tumor characteristics. Stage III disease was present in 44% of patients, with the locally advanced nature of the disease being the cause for conversion to laparotomy in two patients (8%). The technique described allows a lymph node dissection in the chest and the abdomen, but we have not dissected into the porta hepatis. It has not been our practice to do a radical lymphadenectomy. The median number of nodes retrieved from the mediastinum was nine. This is similar to the number (median = 11) we found in our study examining the role of the thoracoscopic-assisted esophagectomy [17]. The median of 11 upper gastric/coelic nodes dissected laparoscopically is the same as we have found at open surgery.
With only collected series and retrospective comparisons available to assess the efficacy of MIE, a compelling argument to support its use would be a demonstrated improvement in quality of life without decrease in disease-free or overall survival. Luketich et al. assessed the issue of quality of life in their series of patients using a validated tool for patients with cancer. They measured the postoperative functional results, in particular reflux, using a tool used most commonly in benign disease assessment. They found that both the mean physical component summary and mental component scores were similar to population normal values. In the group of patients in which pre- and post-operative scores were available, they have reported their patients’ QoL was preserved after a MIE [10].
We found, using the EORTC instruments to assess quality of life, a significant effect from the surgery. There was a reduction in physical function, role function, and global health status that lasted, in some surviving patients, beyond 2 years. Global health status improved above baseline levels, but only in the long term. The EORTC QLQ-OES18 showed deterioration in swallowing (dysphagia scores), eating, GI symptoms (including regurgitation), and cough, with the nadir at 3 months and returning toward baseline at between 12 and 24 months. From these data we believe that the effects on medium-term QoL are a result of the functional aspects of the patient having an esophagectomy and proximal gastrectomy, with the gastric pull-up reconstruction, and not from the approach to the resection. This is clearly the situation in relation to stricture formation and dumping syndrome. Given that the other approaches to esophageal resection offer the same mediastinal dissection and reconstruction, it is difficult to imagine a major improvement in these QoL assessments relating to the access, whether it be MIE or open surgery.
Conclusion
Our group has shown that MIE can be performed safely with morbidity similar to the other approaches. There appears to be a potential for a reduced stay in the hospital by a few days. However, the number of patients in this study was small. MIE remains a significant physiologic trauma for patients, and one questions whether this approach should replace approaches presently used by experienced surgeons with excellent outcomes. Future reports assessing MIE should include QoL assessment as well as the early outcome data to allow a better assessment of whether this approach will be useful for a wider group of esophageal surgeons in the long term.
References
American Joint Committee on Cancer (2003) TNM classification of malignant tumors. 6th ed. 2002. Springer-Verlag, New York
Bailey SH, Bull DA, Harpole DH, Rentz JJ, Neumayer LA, Pappas TN, Daley J, Henderson W, Krasnicka B, Khuri S (2003) Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thoracic Surg 75: 217–222
Bartels H, Thorban S, Siewert JR (1993) Anterior versus posterior reconstruction after transhiatal oesophagectomy: a randomized controlled trial. Br J Surg 80: 1141–1144
Bjordal K, Kaasa S (1992) Psychometric validation of the EORTC Core Quality of Life questionnaire, 30-item version. Acta Oncol 31: 311–321
Blazeby JM, Conroy T, Hammerlid E, Fayers P, Sezer O, Koller M, Arraras J, Bottomley A, Vickery CW, Etienne PL, Alderson D (2003) Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer 39: 1384–1394
DePaula AL, Hashiba K, Ferreira EAB, et al. (1996) Transhiatal approach for esophagectomy. In: Toouli J, Gossot D, Hunter JG, eds. Endosurgery. Churchill Livingstone, New York, 293–299
Ferguson M, Durkin A (2002) Preoperative prediction of the risk of pulmonary complications after esophagectomy for cancer. J Thorac Cardiovasc Surg 123: 661–669
Gluch L, Smith RC, Bambach CP, Brown AR (1999) Comparison of outcomes following transhiatal or Ivor Lewis esophagectomy for esophageal carcinoma. World J Surg 23: 271–276
Jagot P, Sauvanet A, Berthoux L, Belghiti J(1996) Laparoscopic mobilization of the stomach for oesophageal replacement. Br J Surg 83: 540–542
Luketich JD, Alvelo-Rivera M, Buenaventura PO, Christie NA, McCaughan JS, Little VR, Schauer PR, Close JM, Fernando HC (2003) Minimally invasive esophagectomy. Outcomes in 222 patients. Ann Surg 238(4): 486–495
Luketich JD, Nguyen NT, Weigel TL, Ferson P, Keenan R, Schawer P (1998) Minimally invasive approach to esophagectomy. J Soc Laparoendosc Surg 2: 243–247
Muller JM, Erasm H, Stelzner M, Zieren U, Pichlmaier H (1990) Surgical therapy of oesophageal cancer. Br J Surg 77: 845–857
Nguyen NT, Schauer PS, Luketich JD (1999) Combined laparoscopic and thoracoscopic approach to esophagectomy. J Am Coll Surg 188: 329–332
Peracchia A, Rosati R, Fumagalli U, Bona S, Chella B (1997) Thoracoscopic esophagectomy: are there benefits? Semin Surg Oncol 13: 259–262
Rentz J, Bull D, Harpole D, Bailey S, Neumayer L, Pappas T, Krasnicka B, Henderson W, Daley J, Khuri S (2003) Transthoracic versus transhiatal esophagectomy: a prospective study of 945 patients. J Thorac Cardiovasc Surg 125: 1114–1120
Rindani R, Martin CJ, Cox MR (1999) Transhiatal versus Ivor-Lewis oesophagectomy: is there a difference? ANZ J Surg 69: 187–194
Smithers BM, Gotley DC, McEwan D, Martin I, Bessell J, Doyle L (2001) Thoracoscopic mobilization of the esophagus. A 6 year experience. Surg Endosc 15: 176–182
Swanstrom L, Hansen P. (1997) Laparoscopic total esophagectomy. Arch Surg 132: 943–949
Watson DI, Davies N, Jamieson GG (1999) Totally endoscopic Ivor Lewis esophagectomy. Surg Endosc 13: 293–297
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leibman, S., Smithers, B.M., Gotley, D.C. et al. Minimally invasive esophagectomy. Surg Endosc 20, 428–433 (2006). https://doi.org/10.1007/s00464-005-0388-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-005-0388-y