Abstract
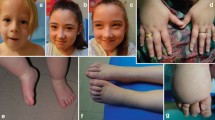
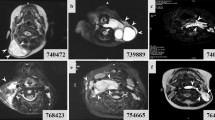
Generalised lymphatic dysplasia (GLD) is characterised by extensive peripheral lymphoedema with visceral involvement. In some cases, it presents in utero with hydrops fetalis. Autosomal dominant and recessive inheritance has been reported. A large, non-consanguineous family with three affected siblings with generalised lymphatic dysplasia is presented. One child died aged 5 months, one spontaneously miscarried at 17 weeks gestation, and the third has survived with extensive lymphoedema. All three presented with hydrops fetalis. There are seven other siblings who are clinically unaffected. Linkage analysis produced two loci on chromosome 18, covering 22 Mb and containing 150 genes, one of which is CCBE1. A homozygous cysteine to serine change in CCBE1 has been identified in the proband, in a residue that is conserved across species. High density SNP analysis revealed homozygosity (a region of 900 kb) around the locus for CCBE1 in all three affected cases. This indicates a likely ancestral mutation that is common to both parents; an example of a homozygous mutation representing Identity by Descent (IBD) in this pedigree. Recent studies in zebrafish have shown this gene to be required for lymphangiogenesis and venous sprouting and are therefore supportive of our findings. In view of the conserved nature of the cysteine, the nature of the amino acid change, the occurrence of a homozygous region around the locus, the segregation within the family, and the evidence from zebrafish, we propose that this mutation is causative for the generalised lymphatic dysplasia in this family, and may be of relevance in cases of non-immune hydrops fetalis.







Similar content being viewed by others
References
Abecasis GR, Cherny SS, Cookson WO, Cardon LR (2002) Merlin-rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet 30:97–101
Al-Gazali LI, Hertecant J, Ahmed R, Khan NA, Padmanabhan R (2003) Further delineation of Hennekam syndrome. Clin Dysmorphol 12:227–232
Angle B, Hersh JH (1997) Expansion of the phenotype in Hennekam syndrome: a case with new manifestations. Am J Med Genet 71:211–214
Bell R, Brice G, Child AH, Murday VA, Mansour S, Sandy CJ, Collin JR, Brady AF, Callen DF, Burnand K, Mortimer P, Jeffery S (2001) Analysis of lymphoedema-distichiasis families for FOXC2 mutations reveals small insertions and deletions throughout the gene. Hum Genet 108:546–551
Brice G, Mansour S, Bell R, Collin JR, Child AH, Brady AF, Sarfarazi M, Burnand KG, Jeffery S, Mortimer P, Murday VA (2002) Analysis of the phenotypic abnormalities in lymphoedema-distichiasis syndrome in 74 patients with FOXC2 mutations or linkage to 16q24. J Med Genet 39:478–483
Cormier-Daire V, Lyonnet S, Lehnert A, Martin D, Salomon R, Patey N, Broyer M, Ricour C, Munnich A (1995) Craniosynostosis and kidney malformation in a case of Hennekam syndrome. Am J Med Genet 57:66–68
Erickson RP, Dagenais SL, Caulder MS, Downs CA, Herman G, Jones MC, Kerstjens-Frederikse WS, Lidral AC, McDonald M, Nelson CC, Witte M, Glover TW (2001) Clinical heterogeneity in lymphoedema-distichiasis with FOXC2 truncating mutations. J Med Genet 38:761–766
Fang J, Dagenais SL, Erickson RP, Arlt MF, Glynn MW, Gorski JL, Seaver LH, Glover TW (2000) Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema-distichiasis syndrome. Am J Hum Genet 67:1382–1388
Ferrell RE, Levinson KL, Esman JH, Kimak MA, Lawrence EC, Barmada MM, Finegold DN (1998) Hereditary lymphedema: evidence for linkage and genetic heterogeneity. Hum Mol Genet 13:2073–2078
Ferrell RE, Kimak MA, Lawrence EC, Finegold DN (2008) Candidate gene analysis in primary lymphedema. Lymphat Res Biol 6:69–76
Forzano F, Faravelli F, Loy A, Di Rocco M (2002) Severe lymphedema, intestinal lymphangiectasia, seizures and mild mental retardation: further case of Hennekam syndrome with a severe phenotype. Am J Med Genet 111:68–70
Hennekam RCM, Geerdink RA, Hamel BCJ, Hennekam FAM, Kraus P, Rammeloo JA, Tillemans AAW (1989) Autosomal recessive intestinal lymphangiectasia and lymphedema, with facial anomalies and mental retardation. Am J Med Genet 34:593–600
Hogan BM, Bos FL, Bussmann J, Witte M, Chi NC, Duckers HJ, Schulte-Merker S (2009) Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat Genet 41:396–398
Irrthum A, Karkkainen MJ, Devriendt K, Alitalo K, Vikkula M (2000) Congenital hereditary lymphedema caused by a mutation that inactivates VEGFR3 tyrosine kinase. Am J Hum Genet 67:295–301
Irrthum A, Devriendt K, Chitayat D, Matthijs G, Glade C, Steijlen PM, Fryns JP, Van Steensel MA, Vikkula M (2003) Mutations in the transcription factor gene SOX18 underlie recessive and dominant forms of hypotrichosis-lymphedema-telangiectasia. Am J Hum Genet 72:1470–1478
Karkkainen MJ, Ferrell RE, Lawrence EC, Kimak MA, Levinson KL, McTigue MA, Alitalo K, Finegold DN (2000) Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat Genet 25:153–159
Makinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, Klein R, Wilkinson GA (2005) PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev 19:397–410
Mortimer PS (1995) Managing lymphoedema. Clin Exp Dermatol 20:98–106
Mortimer PS (1998) The pathophysiology of lymphoedema. Cancer 83:2798–2802
Nagase T, Kikuno R, Ohara O (2001) Prediction of the coding sequences of unidentified human genes. XXII. The complete sequence of 50 new cDNA clones which code for large proteins. DNA Res 8:319–327
Rozen S, Skaletsky HJ (2000) Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S (eds) Bioinformatics methods and protocols: methods in molecular biology. Humana Press, Totowa, pp 365–386
Scarcella A, De Lucia A, Pasquariello MB, Gambardella P (2000) Early death in two sisters with Hennekam syndrome. Am J Med Genet 93:181–183
Tammela T, Petrova TV, Alitalo K (2005) Molecular lymphangiogenesis: new players. TRENDS in Cell Biol 15:434–441
Van Balkom ID, Alders M, Allanson J, Bellini C, Frank U, De Jong G, Kolbe I, Lacombe D, Rockson S, Rowe P, Wijburg F, Hennekam RC (2002) Lymphedema-lymphangiectasia-mental retardation (Hennekam) syndrome: a review. Am J Med Genet 112:412–421
Wigle JT, Oliver G (1999) Prox1 function is required for the development of the murine lymphatic system. Cell 98:769–778
Woods CG, Valente EM, Bond J, Roberts E (2004) A new method for autozygosity mapping using single nucleotide polymorphisms (SNPs) and EXCLUDEAR. J Med Genet 41:101–105
Yasunaga M, Yamanaka C, Mayumi M, Momoi T, Mikawa H (1993) Protein-losing gastroenteropathy with facial anomaly and growth retardation: a mild case of Hennekam syndrome. Am J Med Genet 45:477–480
Acknowledgments
KK, and FC were supported by the British Heart Foundation. PO is supported by the British Skin Foundation. We would like to thank the Reece Spence fund for their support. Much of this work was carried out in the Biomics Unit at SGUL. Members of the Lymphoedema Consortium not given in the author list are: Prof Alberto Smith, Professor Kevin Burnand (St Thomas’) Dr Anne Child (SGUL), Dr Mansoor Sarfarazi (Connecticut). We would like to thank Dr John Crolla and Dr Shuwen Huang (Wessex Regional Genetic Laboratory) for help in analysing the region with the mutation.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
F. Connell, K. Kalidas, P. Ostergaard made equal contributions to this work, and are listed in alphabetical order.
Authors in the consortium are listed in the Acknowledgements.
An erratum to this article can be found at http://dx.doi.org/10.1007/s00439-009-0772-0
Rights and permissions
About this article
Cite this article
Connell, F., Kalidas, K., Ostergaard, P. et al. Linkage and sequence analysis indicate that CCBE1 is mutated in recessively inherited generalised lymphatic dysplasia. Hum Genet 127, 231–241 (2010). https://doi.org/10.1007/s00439-009-0766-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-009-0766-y