Abstract
Pathology reporting is evolving from a traditional narrative report to a more structured synoptic report. Narrative reporting can cause misinterpretation due to lack of information and structure. In this systematic review, we evaluate the impact of synoptic reporting on completeness of pathology reports and quality of pathology evaluation for solid tumours. Pubmed, Embase and Cochrane databases were systematically searched to identify studies describing the effect of synoptic reporting implementation on completeness of reporting and quality of pathology evaluation of solid malignant tumours. Thirty-three studies met the inclusion criteria. All studies, except one, reported an increased overall completeness of pathology reports after introduction of synoptic reporting (SR). Most frequently studied cancers were breast (n = 9) and colorectal cancer (n = 16). For breast cancer, narrative reports adequately described ‘tumour type’ and ‘nodal status’. Synoptic reporting resulted in improved description of ‘resection margins’, ‘DCIS size’, ‘location’ and ‘presence of calcifications’. For colorectal cancer, narrative reports adequately reported ‘tumour type’, ‘invasion depth’, ‘lymph node counts’ and ‘nodal status’. Synoptic reporting resulted in increased reporting of ‘circumferential margin’, ‘resection margin’, ‘perineural invasion’ and ‘lymphovascular invasion’. In addition, increased numbers of reported lymph nodes were found in synoptic reports. Narrative reports of other cancer types described the traditional parameters adequately, whereas for ‘resection margins’ and ‘(lympho)vascular/perineural invasion’, implementation of synoptic reporting was necessary. Synoptic reporting results in improved reporting of clinical relevant data. Demonstration of clinical impact of this improved method of pathology reporting is required for successful introduction and implementation in daily pathology practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ever increasing complexity of cancer treatment requires a high-quality diagnostic process, in which anatomic pathology plays a central role. A complete and clear anatomic pathology report forms the basis for optimal treatment decisions [1]. Depending on cancer type, an increasing number of parameters need to be reported by pathologists [2–5].
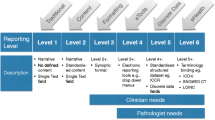
The way anatomic pathology reports are constructed needs to adapt to the continuous increase in complexity of reported diagnostic data [6]. There is a spectrum in the way pathology results are reported. This spectrum is divided into six levels by Srigley et al. [6]. Traditionally, a report consists of the following three paragraphs: macroscopy, microscopy and conclusion all completed with free text and without any further guidelines. These traditional narrative pathology reports (NRs) are considered level 1 reporting. NRs are still the standard in most jurisdictions, even though they are prone to misinterpretation [7] and do not always contain all mandatory information [8–16]. Level three consists of a synoptic-like structured format. With this method, the pathologist follows a checklist per cancer type to ensure that all mandatory parameters are reported. The layout of this type of reporting can still be narrative. More recently, synoptic reporting (SR) has been introduced in pathology. With SR, an electronic reporting module is used with standardised reporting language, multiple-choice answering of mandatory pathology parameters and automated generation of the conclusion (such as TNM stage). Generating a diagnostic report using such a system is much more comparable to filling out a form in an internet browser than it is to narrative reporting using speech recognition software. The result is a well-structured overview of the mandatory parameters for the pathology report (level 6). All levels are described in detail by Srigley et al. [6].
SR has been implemented in several settings all over the world [17]. However, an overview of the effect of SR on the completeness of pathology reports and quality of pathology evaluation in cancer diagnosis is lacking. In the current review, we evaluated the impact of the introduction of SR. We hypothesised that the implementation of SR improved both the completeness of anatomic pathology reports (per parameter and overall) as well as the inherent quality of anatomic pathologic evaluation of cancer specimens.
Materials and methods
To identify studies that described the effect of SR on completeness of reporting and quality of pathology evaluation of solid malignant tumours, a systematic literature search was performed.
Literature search
A combination of search terms in Pubmed, Embase and Cochrane was used to perform the literature search. For the search, we included variations of the following terms: ‘synoptic’, ‘checklist’, ‘template’, ‘pathologic’, ‘histopathology’ and ‘report’. In addition, reference lists of selected papers were manually searched (Online resource 1 describes the search terms in detail). The literature search was performed on September 30, 2015.
Studies were included if studies investigated human subjects, pathology, solid tumours, SR and histology. Selection was first based on title and subsequently on abstract. Only original studies evaluating the effect of SR versus NR of solid malignant tumours were selected. (Conference) abstracts, case reports, editorials, letters and studies for which the full text was not available were excluded. Only studies describing quantitative outcomes of the comparison of SR with NR were included. Therefore, we excluded studies that only described a format of pathology reporting before implementation of SR that described the development of a SR module or the implementation strategy for SR. Two independent investigators (CS and LvL) reviewed each full text report for eligibility.
From each included article, data was extracted on country of study, year and period of study, study design, cancer type, level of reporting before and after the implementation of SR [6], origin of guideline on which the synoptic data parameters are based, outcome measures, results and authors’ conclusion. The format or level of SR as described by Srigley et al. [6] was determined to categorise the studies.
Outcome measures
The outcome measures evaluated in this systematic review were completeness of the pathology reports and the quality of pathology evaluation. We used two definitions for completeness of pathology reports: (1) overall completeness, the proportion of pathology reports containing all mandatory pathology parameters in a given time frame, and (2) parameter-specific completeness, the proportion of pathology reports in which an individual parameter was present in a given time frame. Both definitions were applied to the selected studies.
Quality of pathology evaluation was defined as the proportion of pathology reports in which the informational content corresponds to established quality indicators, such as lymph node numbers, presence of extramural vascular invasion and resection margins.
Data evaluation
The studies were categorised based on cancer type and the implemented level of SR (level 3 versus ≥level 4). To compare completeness, absolute numerical data in studies were converted into percentages. We included parameters that were reported in at least two independent studies. For readability, in the tables, we included only parameters that were reported in at least three independent studies. There is no established definition for sufficient reporting of a parameter. We considered a parameter sufficiently reported if the proportion of pathology reports containing the parameter was greater than 90 % in all the studies that studied the parameter, per cancer type. This percentage was based on definitions used in a number of other studies [18–22].
Results
A total of 3252 potentially relevant studies were retrieved by the database search. After removing duplicates, 2338 studies remained (Fig. 1). We excluded 2156 studies based on title, another 111 studies based on abstract or full text and 38 studies because the full-text article was not available. The remaining 33 studies were included for this review [6, 18–21, 23–52].
Characteristics of studies
Table 1 summarises the characteristics of the 33 included studies. Twenty-three studies had a cross-sectional design and ten a case-control design. The studies originated from the following countries: the UK (n = 7), Australia (n = 6), Canada (n = 5), the USA (n = 4), Norway (n = 4), Germany (n = 2), the Czech Republic, Ireland, Italy, Sri Lanka and Sweden (all n = 1). Ten different types of cancer were covered in the studies. Most covered cancer types were colorectal (n = 16), breast (n = 9) and prostate cancers (n = 6). Twenty-three out of the 33 studies implemented a checklist format (level 3); the other ten studies implemented a higher SR level (≥level 4). Some studies described a two-step process of implementing SR level 4 or higher [6, 18, 23–25, 31, 35, 42, 47]. The SR modules were based on different guidelines, the College of American Pathologists (CAP; n = 12), the Royal College of Pathologists (RCP; n = 9) and other guidelines (n = 5). Some SR modules were based on expert opinion of a pathologist (n = 7).
Completeness of pathology reports
Overall completeness
Out of the 14 studies [21, 23–25, 28, 30, 32, 33, 36, 38, 40, 45–47] that reported the effect of SR on the overall completeness of a pathology report, 13 showed an increased overall completeness, for several cancer types and SR levels (Fig. 2). SR was associated with an increased probability of providing information on the mandatory parameters [23–25] and a decrease in the number of missing parameters in a pathology report [36, 48]. The study that failed to show improved completeness [33] commented on the restricted list of parameters in the SR as defined by CAP. For example, in the guidelines as defined by CAP, SR description of specimen type lacked specific histological codes, whereas in NR, these histological codes could be included.
Impact of synoptic reporting on overall completeness of a pathology report. Fourteen studies [21, 23–25, 28, 30, 32, 33, 36, 38, 40, 45–47] reported the effect of synoptic reporting on the overall completeness of a Pathology report (definition 1). Thirteen studies showed an increased overall completeness, independent of cancer type or synoptic reporting level of the module. In contrast, only one article [33] described that the SR was less complete than the NR
Parameter-specific completeness
Five studies described the impact of SR on parameter-specific completeness in breast cancer. Four studies described the implementation of SR level 3 (Table 2). The results of the fifth article of Branston et al. [28], which implemented SR level 4, were calculated as the percentage change in minimum dataset completeness; these data are excluded from the table. ‘Tumour type’ and ‘lymph node status’ were already reported sufficiently in NR. The ‘oestrogen receptor’ and ‘progesterone receptor’ were already reported sufficiently in NR according to two studies [19, 40], but for another study, implementation of SR was needed to achieve a sufficient reporting [41]. McEvoy et al. reported increased completeness of the oestrogen receptor from 84 to 99 %; however, a decrease was seen for the progesterone receptor [41]. The implementation of SR led to an increased completeness of four parameters (‘resection margins’, ‘DCIS size’, ‘location: quadrant’ and ‘calcification’). Three parameters increased significantly in the majority of the studies ‘histological grade’ [19, 23, 40, 41], ‘lymphovascular invasion’ [19, 23, 41] and ‘lesion size’ [19, 23] or already showed sufficient completeness in NR [19, 40]. The parameters ‘distance tumour to resection margin’, ‘type of specimen’, ‘location side’, ‘multiple tumour foci’ and ‘CIS in specimen’ showed diverse results; in some studies, the parameters were already sufficiently reported in NR, whilst in other studies, implementation of SR was necessary.
Fourteen studies on SR of colorectal cancer described a quantitative effect on parameter-specific completeness. Of these 14 studies, 13 are represented in Table 3. For colorectal cancer, we merged colon and rectal cancer data if reported separately. The results of the 14th article by Branston et al. [28], which implemented SR level 4, were calculated as the percentage change in minimum dataset completeness. These were excluded from the table. Nine studies described the effect of implementing SR level 3, and five studies described the effect of implementing SR level 4 or higher. Four individual parameters were already reported sufficiently in NR (tumour type (Fig. 3a), ‘depth of invasion’, ‘total lymph nodes’ and ‘lymph nodes with metastasis’). ‘Tumour size’ was adequately reported in the NR of three studies [18, 42, 46] but lacking in a fourth [21]. ‘Histological grade’ was sufficiently reported in the majority of studies (n = 9) but not in three other studies [26, 28, 36]. The completeness of both parameters was increased to 96–100 % after the introduction of SR. The implementation of SR led to increased completeness for the reporting of the ‘circumferential resection margin’ (Fig. 3b), ‘distant resection margins’, ‘perineural invasion’ and ‘vascular and lymphovascular invasion’. The parameters ‘stage’, ‘resection margin’ and ‘nodal status’ showed diverse results; in some studies, NR was already very good, whilst in other studies, the implementation of SR was necessary.
Impact of synoptic reporting on individual parameters in a colorectal specimen pathology report. a The effect of synoptic reporting on the proportion of pathology reports containing information on tumour type in colorectal cancers. b The effect of synoptic reporting on the proportion of pathology reports containing information on circumferential margin in rectal cancers. c The effect of synoptic reporting on the absolute mean number of lymph nodes resected per resection specimen. d The effect of synoptic reporting on the proportion of pathology reports reported 12 or more lymph nodes resected
Eight studies on SR described other cancer types, as shown in the tables (Online resources 2–6). Common parameters ‘tumour size’, ‘histological type’ and ‘histological grade’ were already reported sufficiently in NR, whereas for ‘resection margins’ and ‘(lympho)vascular/perineural invasion’, implementation of SR was necessary for an increased completeness to 96–100 %.
Quality of pathology evaluation
Implementation of SR is also expected to affect the quality of pathology evaluation. One aspect of quality is the accurate ascertainment of nodal tumour metastasis. If more lymph nodes are being resected, the N stage will be reported more accurately. For colorectal cancer, it is advised internationally to resect at least 12 lymph nodes [53]. The mean number of lymph nodes identified in the surgical specimen for colorectal cancer was evaluated in 5 of the 14 included studies [18, 29, 37, 46, 51]. All studies showed improvement in mean number of lymph nodes after implementation of SR (Fig. 3c), and more frequently, the minimum number of 12 lymph nodes was achieved. Three studies also showed an improvement of the proportion of pathology reports with a minimum of 12 lymph nodes reported after implementation of SR (Fig. 3d).
Discussion
In this systematic review, we showed that SR results in more complete pathology reports. Whilst traditional parameters such as ‘tumour type’, ‘grade’, ‘invasion depth’ and ‘nodal status’ are in general well reported with NR, other clinical relevant features such as resection margins and ‘type of local spread (vascular, lymphovascular and perineural invasion)’ are frequently lacking. The introduction of SR results in improved reporting of these parameters. SR also improves the mean number of lymph nodes reported and the proportion of pathology reports with 12 or more lymph nodes [53].
Besides these favourable quantitative outcomes, pathologists found that SR was quick and easy to complete and that reports included all essential parameters [28]. Even though SR appears to be more time-consuming in the beginning, implementation actually resulted in a significant reduction time spent on the production of the report by pathologists [54, 55]. For multidisciplinary meetings, both pathologists and clinicians appreciated consistency of the reports [56]. Necessary information for patient management was quick and unambiguous to find [28, 56].
SR can be implemented in different ways. In the studies included in the present review, the following six different implementation strategies were described: combined implementation of SR with clinical audits [23, 31, 47], organisation of SR education or meetings [18, 28, 32, 37], attachment of SR hard copy to the request form of the resection specimen [21, 31, 44], addition of explanatory notes to the SR [20, 24], mandated inclusion of essential parameters according to guidelines [33, 36, 51] or introduction of the SR module without any special attention [19, 26, 29, 30, 38, 41, 42, 46]. The implementation strategy could partially explain the success of implementation of SR. Srigley et al. described the implementation of SR in Ontario, Canada, where pilots and audits were used to ensure proper implementation of SR. In 2012, they achieved successful implementation in 92 % of all hospitals in Ontario [6]. In addition, funding for hospitals, as was used in Ontario [6, 47], could also have added to the successful implementation of SR.
To date, SR has not been widely adopted in anatomic pathology reporting. The main barriers preventing successful implementation are the personal preference of pathologists, who like the flexibility and work flow of NR [57]. Whilst indeed initially, introduction is likely to disrupt the work flow, this seems a temporary situation. Flexibility is sometimes necessary to express uncertainty about a diagnosis; this can in most cases be solved by addition of free text fields to a SR. For instance, Hassel et al. [58] reported that pathologists found the SR more difficult and inflexible and they missed parameters. Another factor hampering implementation is the introduction of the new reporting format in existing work environments, such as the electronic patient files and software systems used throughout the hospital [57, 59]. As reported by Bjugn et al. [27], the development of the SR in Norway was delayed considerably because of alterations in the mandatory diagnostic criteria of the SR and because of alterations in the user interface for the SR.
There are some potential limitations to our study. We are confident that with our search, we found the majority of published papers, minimising the risk of selection bias. However, publication bias may cause an overrepresentation of positive study outcomes.
All studies in this review were observational. The design was either case-control or cross-sectional. No randomised controlled trial has been conducted on the effect of SR on pathology reporting. However, in our opinion, a retrospective study is suitable to investigate the effect of SR in practice. Eight studies reported the effect of SR in less than 200 reports [20, 21, 23, 37, 40, 43, 46, 48]; this is partly due to manually auditing the data for completeness. Preferably, future studies would include much higher number of pathology reports to get a better understanding of the impact of SR on pathology reporting. The fact that these studies cover different tumour types and are conducted in different countries and continents increases the generalisability. Even though most articles investigated the effect of breast and/or colon cancer, we expect that the results reported in this review are transferable to implementation of SR for other cancer types and countries not yet investigated.
Based on the current data, we can conclude that SR results in improved reporting of clinical relevant data. For this reason, it is our opinion that SR is already at present the best clinical practice for anatomic pathology cancer reporting. Ongoing innovation in SR software will likely further improve the value of SR in anatomic pathology, as well as improve the ease of use and efficiency of reporting with SR modules.
References
Nakhleh RE (2011) Quality in surgical pathology communication and reporting. Arch Pathol Lab Med 135(11):1394–1397. doi:10.5858/arpa.2011-0192-RA
Pathologists CoA cancer protocol templates. 2016 http://www.cap.org/web/home/resources/cancer-reporting-tools
Pathologists tRCo (2015) Datasets and tissue pathways. http://www.rcpath.org/publications-media/publications/datasets. Accessed 11–05-2015 2015
Cancer AJCo AJCC cancer staging. https://cancerstaging.org/Pages/default.aspx. Accessed 11–05-2015 2015
Control UfIC. http://www.uicc.org/. Accessed 11–05-2015 2015
Srigley J, Lankshear S, Brierley J, McGowan T, Divaris D, Yurcan M, Rossi R, Yardley T, King MJ, Ross J, Irish J, McLeod R, Sawka C (2013) Closing the quality loop: facilitating improvement in oncology practice through timely access to clinical performance indicators. J Oncol Pract 9(5):e255–e261. doi:10.1200/JOP.2012.000818
Powsner SM, Costa J, Homer RJ (2000) Clinicians are from Mars and pathologists are from Venus. Arch Pathol Lab Med 124(7):1040–1046. doi:10.1043/0003-9985(2000)124<1040:CAFMAP>2.0.CO;2
Ali Shah SA, Amer Syed M (2013) An audit of colorectal cancer histopathology reports in a tertiary care hospital. J Med Sci (Peshawar) 21(3):128–130
Atanda AT, Atanda JO (2010) Audit of histopathology reports for breast cancer in Aminu Kano Teaching hospital (Verification des rapports histopathologie du cancer du sein dans un hopital d’enseignement Aminu Kano.). West Afr J Med 29(3):174–177
Chatelain D, Farges O, Fuks D, Trouillet N, Pruvot FR, Regimbeau JM (2012) Assessment of pathology reports on hilar cholangiocarcinoma: the results of a nationwide, multicenter survey performed by the AFC-HC-2009 study group. J Hepatol 56(5):1121–1128. doi:10.1016/j.jhep.2011.12.010
Eon Y, Le Douy JY, Lamer B, Battini J, Bretagne JF (2006) Quality and completeness of histopathology reports of rectal cancer resections. results of an audit in Brittany. Gastroenterol Clin Biol 30(2):235–240
Gephardt GN, Baker PB (1996) Lung carcinoma surgical pathology report adequacy: a College of American Pathologists Q-Probes study of over 8300 cases from 464 institutions. Arch Pathol Lab Med 120(10):922–927
Isidro ML, Lugo G, Fidalgo O, Garcia-Arias S (2012) Adequacy of pathology reports of specimens from patients with differentiated thyroid cancer. Endocr Pathol 23(4):215–220. doi:10.1007/s12022-012-9226-7
King PM, Blazeby JM, Gupta J, Alderson D, Moorghen M (2004) Upper gastrointestinal cancer pathology reporting: a regional audit to compare standards with minimum datasets. J Clin Pathol 57(7):702–705. doi:10.1136/jcp.2003.013326
Nambiar A, Vivek N, Bindu MR, Sudheer OV, Bai L (2010) Completeness of low anterior resection pathology report: a hospital-based audit with recommendations on improving reporting. Indian J Cancer 47(2):156–159. doi:10.4103/0019-509x.63010
Silcocks P, Needham P, Hemsley F (1999) Audit of prostate cancer: lessons learnt for current clinical practice, surrogates for quality of care and standardisation and quality assurance. Public Health 113(4):161–164. doi:10.1016/S0033-3506%2899%2900146-8
Williams CL, Bjugn R, Hassell LA (2015) Current status of discrete data capture in synoptic surgical pathology and cancer reporting. Pathol Lab Med Int 7:11–22
Chan NG, Duggal A, Weir MM, Driman DK (2008) Pathological reporting of colorectal cancer specimens: a retrospective survey in an academic Canadian pathology department. Can J Surg 51(4):284–288
Austin R, Thompson B, Coory M, Walpole E, Francis G, Fritschi L (2009) Histopathology reporting of breast cancer in Queensland: the impact on the quality of reporting as a result of the introduction of recommendations. Pathology 41(4):361–365. doi:10.1080/00313020902884469
Gill AJ, Johns AL, Eckstein R, Samra JS, Kaufman A, Chang DK, Merrett ND, Cosman PH, Smith RC, Biankin AV, Kench JG (2009) Synoptic reporting improves histopathological assessment of pancreatic resection specimens. Pathology 41(2):161–167. doi:10.1080/00313020802337329
Siriwardana PN, Pathmeswaran A, Hewavisenthi J, Deen KI (2009) Histopathology reporting in colorectal cancer: a proforma improves quality. Colorectal Disease 11(8):849–853. doi:10.1111/j.1463-1318.2008.01680.x
Pathologists TRCo (2014) Standards and datasets for reporting cancers. dataset for colorectal cancer histopathology reports. The Royal College of Pathologists, London
Appleton MA, Douglas-Jones AG, Morgan JM (1998) Evidence of effectiveness of clinical audit in improving histopathology reporting standards of mastectomy specimens. J Clin Pathol 51(1):30–33
Aumann K, Amann D, Gumpp V, Hauschke D, Kayser G, May AM, Wetterauer U, Werner M (2012) Template-based synoptic reports improve the quality of pathology reports of prostatectomy specimens. Histopathology 60(4):634–644. doi:10.1111/j.1365-2559.2011.04119.x
Aumann K, Kayser G, Amann D, Bronsert P, Hauschke D, Palade E, Passlick B, Werner M (2013) The format type has impact on the quality of pathology reports of oncological lung resection specimens. Lung Cancer 81(3):382–387. doi:10.1016/j.lungcan.2013.05.017
Beattie GC, McAdam TK, Elliott S, Sloan JM, Irwin ST (2003) Improvement in quality of colorectal cancer pathology reporting with a standardized proforma—a comparative study. Colorectal Disease 5(6):558–562. doi:10.1046/j.1463-1318.2003.00466.x
Bjugn R, Casati B, Norstein J (2008) Structured electronic template for histopathology reports on colorectal carcinomas: a joint project by the Cancer Registry of Norway and the Norwegian Society for Pathology. Hum Pathol 39(3):359–367. doi:10.1016/j.humpath.2007.06.019
Branston LK, Greening S, Newcombe RG, Daoud R, Abraham JM, Wood F, Dallimore NS, Steward J, Rogers C, Williams GT (2002) The implementation of guidelines and computerised forms improves the completeness of cancer pathology reporting. the CROPS project: a randomised controlled trial in pathology. Eur J Cancer (Oxford, England: 1990) 38(6):764–772
Buchwald P, Olofsson F, Lorinc E, Syk I (2011) Standard protocol for assessment of colon cancer improves the quality of pathology. Color Dis 13(3):e33–e36. doi:10.1111/j.1463-1318.2010.02454.x
Casati B, Bjugn R (2012) Structured electronic template for histopathology reporting on colorectal carcinoma resections: five-year follow-up shows sustainable long-term quality improvement. Arch Pathol Lab Med 136(6):652–656. doi:10.5858/arpa.2011-0370-OA
Cross SS, Feeley KM, Angel CA (1998) The effect of four interventions on the informational content of histopathology reports of resected colorectal carcinomas. J Clin Pathol 51(6):481–482
Hammond EH, Flinner RL (1997) Clinically relevant breast cancer reporting: using process measures to improve anatomic pathology reporting. Arch Pathol Lab Med 121(11):1171–1175
Hassell L, Aldinger W, Moody C, Winters S, Gerlach K, Schwenn M, Perriello D (2009) Electronic capture and communication of synoptic cancer data elements from pathology reports: results of the reporting pathology Protocols 2 (RPP2) project. J Registry Manag 36(4):117–124 quiz 163-115
Haugland HK, Casati B, Dorum LM, Bjugn R (2011) Template reporting matters—a nationwide study on histopathology reporting on colorectal carcinoma resections. Human Pathol 42(1):36–40. doi:10.1016/j.humpath.2010.06.009
Haydu LE, Holt PE, Karim RZ, Madronio CM, Thompson JF, Armstrong BK, Scolyer RA (2010) Quality of histopathological reporting on melanoma and influence of use of a synoptic template. Histopathology 56(6):768–774. doi:10.1111/j.1365-2559.2010.03546.x
Idowu MO, Bekeris LG, Raab S, Ruby SG, Nakhleh RE (2010) Adequacy of surgical pathology reporting of cancer: a College of American Pathologists Q-Probes study of 86 institutions. Arch Pathol Lab Med 134(7):969–974. doi:10.1043/2009-0412-cp.1
Ihnat P, Delongova P, Horacek J, Ihnat Rudinska L, Vavra P, Zonca P (2014) The impact of standard protocol implementation on the quality of colorectal cancer pathology reporting. World J Surg. doi:10.1007/s00268-014-2796-4
Kahn C, Simonella L, Sywak M, Boyages S, Ung O, O’Connell D (2012) Postsurgical pathology reporting of thyroid cancer in new South Wales, Australia. Thyroid 22(6):604–610. doi:10.1089/thy.2011.0501
Karim RZ, Van Den Berg KS, Colman MH, McCarthy SW, Thompson JF, Scolyer RA (2008) The advantage of using a synoptic pathology report format for cutaneous melanoma. Histopathology 52(2):130–138. doi:10.1111/j.1365-2559.2007.02921.x
Mathers ME, Shrimankar J, Scott DJ, Charlton FG, Griffith CDM, Angus B (2001) The use of a standard proforma in breast cancer reporting. J Clin Pathol 54(10):809–811
McEvoy SP, Ingram DM, Byrne MJ, Joseph DJ, Dewar J, Trotter J, Harper C, Haworth C, Harvey JM, Sterrett GF, Jamrozik K, Fritschi L (2004) Breast cancer in Western Australia: clinical practice and clinical guidelines. Med J Aust 181(6):305–309
Messenger DE, McLeod RS, Kirsch R (2011) What impact has the introduction of a synoptic report for rectal cancer had on reporting outcomes for specialist gastrointestinal and nongastrointestinal pathologists? Arch Pathol Lab Med 135(11):1471–1475. doi:10.5858/arpa.2010-0558-OA
Porter GA, Urquhart RL, Rheaume D, Cwajna S, Cox MA, Grunfeld E (2013) Clinical information available to oncologists in surgically treated rectal cancer: room to improve. Curr Oncol 20(3):166–172. doi:10.3747/co.20.1215
Reid WA, al-Nafussi AI, Rebello G, Williams AR (1999) Effect of using templates on the information included in histopathology reports on specimens of uterine cervix taken by loop excision of the transformation zone. J Clin Pathol 52(11):825–828
Renshaw SA, Mena-Allauca M, Touriz M, Renshaw A, Gould EW (2014) The impact of template format on the completeness of surgical pathology reports. Arch Pathol Lab Med 138(1):121–124. doi:10.5858/arpa.2012-0733-OA
Rigby K, Brown SR, Lakin G, Balsitis M, Hosie KB (1999) The use of a proforma improves colorectal cancer pathology reporting. Ann R Coll Surg Engl 81(6):401–403
Srigley JR, McGowan T, Maclean A, Raby M, Ross J, Kramer S, Sawka C (2009) Standardized synoptic cancer pathology reporting: a population-based approach. J Surg Oncol 99(8):517–524. doi:10.1002/jso.21282
Ventura L, De Vito M, Leocata P, Ventura T (2003) An original protocol for standardized histopathology reporting of prostate carcinoma. Arch Ital Urol Androl 75(4):208–213
Westgaard A, Laronningen S, Mellem C, Eide TJ, Clausen OPF, Moller B, Gladhaug IP (2009) Are survival predictions reliable? Hospital volume versus standardisation of histopathologic reporting for accuracy of survival estimates after pancreatoduodenectomy for adenocarcinoma. Eur J Cancer 45(16):2850–2859. doi:10.1016/j.ejca.2009.03.019
Westgaard A, Clausen OPF, Gladhaug IP (2011) Survival estimates after pancreatoduodenectomy skewed by non-standardized histopathology reports. Apmis 119(10):689–700. doi:10.1111/j.1600-0463.2011.02783.x
Woods YL, Mukhtar S, McClements P, Lang J, Steele RJ, Carey FA (2014) A survey of reporting of colorectal cancer in Scotland: compliance with guidelines and effect of proforma reporting. J Clin Pathol 67(6):499–505. doi:10.1136/jclinpath-2013-202060
Zarbo RJ (1992) Interinstitutional assessment of colorectal carcinoma surgical pathology report adequacy. A College of American Pathologists Q-Probes study of practice patterns from 532 laboratories and 15,940 reports. Arch Pathol Lab Med 116(11):1113–1119
Compton CC, Greene FL (2004) The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin 54(6):295–308
Murari M, Pandey R (2006) A synoptic reporting system for bone marrow aspiration and core biopsy specimens. Arch Pathol Lab Med 130(12):1825–1829. doi:10.1043/1543-2165(2006)130[1825:ASRSFB]2.0.CO;2
Yunker WK, Matthews TW, Dort JC (2008) Making the most of your pathology: standardized histopathology reporting in head and neck cancer. J Otolaryngol Head Neck Surg 37(1):48–55
Lankshear S, Srigley J, McGowan T, Yurcan M, Sawka C (2013) Standardized synoptic cancer pathology reports—so what and who cares? A population-based satisfaction survey of 970 pathologists, surgeons, and oncologists. Arch Pathol Lab Med 137(11):1599–1602. doi:10.5858/arpa.2012-0656-OA
services UDohah (2009) Electronic reporting in pathology: requirements and limitations. A Paradigm for National Electronic Health Records Implementation, Washington, DC
Hassell LA, Parwani AV, Weiss L, Jones MA, Ye J (2010) Challenges and opportunities in the adoption of College of American Pathologists checklists in electronic format: perspectives and experience of reporting pathology Protocols project (RPP2) participant laboratories. Arch Pathol Lab Med 134(8):1152–1159. doi:10.1043/2009-0386-oa.1
Casati B, Haugland HK, Barstad GM, Bjugn R (2014) Factors affecting the implementation and use of electronic templates for histopathology cancer reporting. Pathology 46(3):165–168. doi:10.1097/PAT.0000000000000065
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This study was funded by Dutch Cancer Society/Alpe d’huzes (grant number KUN 2013–6354).
Human and animal rights
Not applicable.
Electronic supplementary material
Supplementary information is available at Virchows Archive website.
ESM 1
(DOCX 43 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sluijter, C.E., van Lonkhuijzen, L.R.C.W., van Slooten, HJ. et al. The effects of implementing synoptic pathology reporting in cancer diagnosis: a systematic review. Virchows Arch 468, 639–649 (2016). https://doi.org/10.1007/s00428-016-1935-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-016-1935-8