Abstract
There is increasing recognition of the heterogeneity of origin of cases of autism spectrum disorder (ASD) with multiple forms of ASD having been identified over the decades. Among these, a genetic etiology can be identified in 20–40% of cases when a full genetic work-up is completed. The Fragile X premutation state (characterized by the presence of 55–200 CGG repeats in the FMR1 gene) is a relatively newly identified disease state that has since been associated with several disorders including fragile X-associated tremor ataxia syndrome (FXTAS), fragile X-associated primary ovarian insufficiency (FXPOI) and most recently, fragile X-associated neurodevelopmental disorders (FXAND) which commonly includes anxiety and depression. In addition to these associated disorders, extant literature and clinical observations have suggested an association between the premutation state and ASD. In this paper, we review the literature pertinent to this and discuss possible molecular mechanisms that may explain this association. This includes lowered levels of the FMR1 Protein (FMRP), GABA deficits, mitochondrial dysfunction and secondary genetic abnormalities that is seen in premutation carriers as well as their increased vulnerability to environmental stressors. Understanding these mechanisms can facilitate development of targeted treatment for specific sub-groups of ASD and premutation disorders in future.

Similar content being viewed by others
Data availability
Not Applicable.
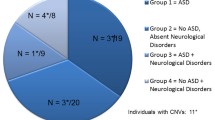
Change history
20 October 2022
Typographical error in Figure 1. The word ‘increased’ in the box in the right hand side of the figure 1 is spelt wrongly and it has been updated.
Abbreviations
- ASD:
-
Autism spectrum disorder
- FXS:
-
Fragile X syndrome
- FMRP:
-
FMR1 Protein
- PM:
-
Premutation
- FMR1 :
-
Fragile X messenger ribonucleoprotein 1 gene
References
Association D-AP (2013) Diagnostic and statistical manual of mental disorders. American Psychiatric Publishing, Arlington
Maenner MJ et al (2021) Prevalence and characteristics of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 Sites, United States, 2018. MMWR Surveill Summ 70(11):1–16
Baxter AJ et al (2015) The epidemiology and global burden of autism spectrum disorders. Psychol Med 45(3):601–613
Tammimies K et al (2015) Molecular diagnostic yield of chromosomal microarray analysis and whole-exome sequencing in children with autism spectrum disorder. JAMA 314(9):895–903
Hyman SL, Levy SE, Myers SM, Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics, 2020. 145(1).
Bagni C, Zukin RS (2019) A synaptic perspective of fragile X syndrome and autism spectrum disorders. Neuron 101(6):1070–1088
Kaufmann WE et al (2017) Autism spectrum disorder in fragile X syndrome: cooccurring conditions and current treatment. Pediatrics 139(Suppl 3):S194-s206
Tassone F et al (2012) FMR1 CGG allele size and prevalence ascertained through newborn screening in the United States. Genome Med 4(12):100
Saldarriaga W et al (2014) Fragile X syndrome. Colomb Med (Cali) 45(4):190–198
Bagni C et al (2012) Fragile X syndrome: causes, diagnosis, mechanisms, and therapeutics. J Clin Invest 122(12):4314–4322
Verkerk AJ et al (1991) Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65(5):905–914
Tassone F et al (2000) Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet 66(1):6–15
Cronister A et al (1991) Heterozygous fragile X female: historical, physical, cognitive, and cytogenetic features. Am J Med Genet 38(2–3):269–274
Sullivan AK et al (2005) Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod 20(2):402–412
Hagerman RJ et al (2001) Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology 57(1):127–130
Jacquemont S et al (2003) Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet 72(4):869–878
Jacquemont S et al (2004) Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA 291(4):460–469
Hagerman RJ, Hagerman P (2016) Fragile X-associated tremor/ataxia syndrome - features, mechanisms and management. Nat Rev Neurol 12(7):403–412
Hagerman RJ et al (2018) Fragile X-associated neuropsychiatric disorders (FXAND). Front Psychiatry 9:564
Roberts JE et al (2009) Mood and anxiety disorders in females with the FMR1 premutation. Am J Med Genet B Neuropsychiatr Genet 150B(1):130–139
Roberts JE et al (2016) Trajectory and predictors of depression and anxiety disorders in mothers with the FMR1 premutation. Biol Psychiatry 79(10):850–857
Sobesky WE et al (1994) Emotional and neurocognitive deficits in fragile X. Am J Med Genet 51(4):378–385
Chonchaiya W et al (2012) Increased prevalence of seizures in boys who were probands with the FMR1 premutation and co-morbid autism spectrum disorder. Hum Genet 131(4):581–589
Bailey DB Jr et al (2008) Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. Am J Med Genet A 146A(16):2060–2069
Farzin F et al (2006) Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr 27(2 Suppl):S137–S144
Clifford S et al (2007) Autism spectrum phenotype in males and females with fragile X full mutation and premutation. J Autism Dev Disord 37(4):738–747
Baron-Cohen S et al (2001) The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord 31(1):5–17
White SJ et al (2021) Autistic traits and mental health in women with the fragile-X premutation: maternal status versus genetic risk. Br J Psychiatry 218(1):28–34
Losh M et al (2012) Defining genetically meaningful language and personality traits in relatives of individuals with fragile X syndrome and relatives of individuals with autism. Am J Med Genet B Neuropsychiatr Genet 159B(6):660–668
Maltman N et al (2021) The phenotypic profile associated with the FMR1 premutation in women: an investigation of clinical-behavioral, social-cognitive, and executive abilities. Front Psychiatry 12:718485
Baker EK et al (2019) Incomplete silencing of full mutation alleles in males with fragile X syndrome is associated with autistic features. Mol Autism 10:21
Harris HK et al. Pathogenic Yield of Genetic Testing in Autism Spectrum Disorder. Pediatrics, 2020. 146(4).
Pinto D et al (2014) Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am J Hum Genet 94(5):677–694
De Rubeis S et al (2014) Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515(7526):209–215
O’Roak BJ et al (2012) Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485(7397):246–250
Betancur C, Buxbaum JD (2013) SHANK3 haploinsufficiency: a “common” but underdiagnosed highly penetrant monogenic cause of autism spectrum disorders. Mol Autism 4(1):17
Monteiro P, Feng G (2017) SHANK proteins: roles at the synapse and in autism spectrum disorder. Nat Rev Neurosci 18(3):147–157
Sanders SJ et al (2015) Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron 87(6):1215–1233
Bain JM, Hagerman R J, Pediatric and neurological assessments, in textbook of autism spectrum disorders, E. Hollander, R. Hagerman, and C. Ferretti, Editors. 2022, American Psychiatric Association Publishing: Washington, DC. p. 87–99.
Hagerman RJ, Hagerman PJ (2008) Testing for fragile X gene mutations throughout the life span. JAMA 300(20):2419–2421
Borrie SC et al (2017) Cognitive dysfunctions in intellectual disabilities: the contributions of the Ras-MAPK and PI3K-AKT-mTOR Pathways. Annu Rev Genomics Hum Genet 18:115–142
Huber KM et al (2015) Dysregulation of mammalian target of rapamycin signaling in mouse models of autism. J Neurosci 35(41):13836–13842
Subramanian M et al (2015) Characterizing autism spectrum disorders by key biochemical pathways. Front Neurosci 9:313
Ludwig AL et al (2014) CNS expression of murine fragile X protein (FMRP) as a function of CGG-repeat size. Hum Mol Genet 23(12):3228–3238
Pretto DI et al (2015) Differential increases of specific FMR1 mRNA isoforms in premutation carriers. J Med Genet 52(1):42–52
Napoli E et al (2018) Impact of FMR1 premutation on neurobehavior and bioenergetics in young monozygotic twins. Front Genet 9:338
Riddle JE et al (1998) Phenotypic involvement in females with the FMR1 gene mutation. Am J Ment Retard 102(6):590–601
Bleuzé L, Triaca V, Borreca A (2021) FMRP-driven neuropathology in autistic spectrum disorder and alzheimer’s disease: a losing game. Front Mol Biosci 8:867
Davidovic L et al (2007) The fragile X mental retardation protein is a molecular adaptor between the neurospecific KIF3C kinesin and dendritic RNA granules. Hum Mol Genet 16(24):3047–3058
Dictenberg JB et al (2008) A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile X syndrome. Dev Cell 14(6):926–939
Iossifov I et al (2012) De novo gene disruptions in children on the autistic spectrum. Neuron 74(2):285–299
Masini E et al. An overview of the main genetic, epigenetic and environmental factors involved in autism spectrum disorder focusing on synaptic activity. Int J Mol Sci, 2020. 21(21).
Waye MMY, Cheng HY (2018) Genetics and epigenetics of autism: a review. Psychiatry Clin Neurosci 72(4):228–244
Hagerman R, Hoem G, Hagerman P (2010) Fragile X and autism: intertwined at the molecular level leading to targeted treatments. Mol Autism 1(1):12
Fatemi SH et al (2013) Impairment of fragile X mental retardation protein-metabotropic glutamate receptor 5 signaling and its downstream cognates ras-related C3 botulinum toxin substrate 1, amyloid beta A4 precursor protein, striatal-enriched protein tyrosine phosphatase, and homer 1, in autism: a postmortem study in cerebellar vermis and superior frontal cortex. Mol Autism 4(1):21
Fatemi SH, Folsom TD (2011) The role of fragile X mental retardation protein in major mental disorders. Neuropharmacology 60(7–8):1221–1226
Fatemi SH et al (2010) Fragile X mental retardation protein levels are decreased in major psychiatric disorders. Schizophr Res 124(1–3):246–247
Fatemi SH et al (2009) Expression of GABA(B) receptors is altered in brains of subjects with autism. Cerebellum 8(1):64–69
Fatemi SH et al (2010) mRNA and protein levels for GABAAalpha4, alpha5, beta1 and GABABR1 receptors are altered in brains from subjects with autism. J Autism Dev Disord 40(6):743–750
Ghose S et al (2011) The GABAβ receptor as a target for antidepressant drug action. Br J Pharmacol 162(1):1–17
Duncan CE et al (2010) Prefrontal GABA(A) receptor alpha-subunit expression in normal postnatal human development and schizophrenia. J Psychiatr Res 44(10):673–681
D’Hulst C et al (2006) Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res 1121(1):238–245
Gantois I et al (2006) Expression profiling suggests underexpression of the GABA(A) receptor subunit delta in the fragile X knockout mouse model. Neurobiol Dis 21(2):346–357
Fatemi SH et al (2011) Metabotropic glutamate receptor 5 upregulation in children with autism is associated with underexpression of both Fragile X mental retardation protein and GABAA receptor beta 3 in adults with autism. Anat Rec (Hoboken) 294(10):1635–1645
Yang Y et al (2013) Reactive oxygen species in the immune system. Int Rev Immunol 32(3):249–270
Terzi A, Suter DM (2020) The role of NADPH oxidases in neuronal development. Free Radic Biol Med 154:33–47
Yun HR, et al. Roles of Autophagy in Oxidative Stress. Int J Mol Sci, 2020. 21(9).
Valor LM, et al. Molecular pathogenesis and peripheral monitoring of adult fragile X-associated syndromes. Int J Mol Sci, 2021. 22(16).
Song G et al (2016) Altered redox mitochondrial biology in the neurodegenerative disorder fragile X-tremor/ataxia syndrome: use of antioxidants in precision medicine. Mol Med 22:548–559
Srinivas US et al (2019) ROS and the DNA damage response in cancer. Redox Biol 25:101084
Singh, A., et al., Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules, 2019. 24(8).
Kaplan ES et al (2012) Early mitochondrial abnormalities in hippocampal neurons cultured from Fmr1 pre-mutation mouse model. J Neurochem 123(4):613–621
Todd PK et al (2013) CGG repeat-associated translation mediates neurodegeneration in fragile X tremor ataxia syndrome. Neuron 78(3):440–455
Napoli E et al (2011) Altered zinc transport disrupts mitochondrial protein processing/import in fragile X-associated tremor/ataxia syndrome. Hum Mol Genet 20(15):3079–3092
Napoli E et al (2016) Altered bioenergetics in primary dermal fibroblasts from adult carriers of the FMR1 premutation before the onset of the neurodegenerative disease fragile X-associated tremor/ataxia syndrome. Cerebellum 15(5):552–564
Ross-Inta C et al (2010) Evidence of mitochondrial dysfunction in fragile X-associated tremor/ataxia syndrome. Biochem J 429(3):545–552
Nobile V et al (2020) Altered mitochondrial function in cells carrying a premutation or unmethylated full mutation of the FMR1 gene. Hum Genet 139(2):227–245
Loesch DZ et al (2017) Novel blood biomarkers are associated with white matter lesions in fragile x- associated tremor/ataxia syndrome. Neurodegener Dis 17(1):22–30
Robin G et al (2017) Calcium dysregulation and Cdk5-ATM pathway involved in a mouse model of fragile X-associated tremor/ataxia syndrome. Hum Mol Genet 26(14):2649–2666
Ariza J et al (2015) Dysregulated iron metabolism in the choroid plexus in fragile X-associated tremor/ataxia syndrome. Brain Res 1598:88–96
Napoli E et al (2016) Premutation in the fragile X mental retardation 1 (FMR1) gene affects maternal Zn-milk and perinatal brain bioenergetics and scaffolding. Front Neurosci 10:159
Napoli E et al (2020) Characterization of the Metabolic, Clinical and Neuropsychological Phenotype of Female Carriers of the Premutation in the X-Linked FMR1 Gene. Front Mol Biosci 7:578640
Giulivi C et al (2016) Plasma biomarkers for monitoring brain pathophysiology in FMR1 premutation carriers. Front Mol Neurosci 9:71
Giulivi C et al (2010) Mitochondrial dysfunction in autism. JAMA 304(21):2389–2396
Siddiqui MF, Elwell, and Johnson MH, Mitochondrial dysfunction in autism spectrum disorders. Autism Open Access, 2016. 6(5).
Saxena R et al (2020) Role of environmental factors and epigenetics in autism spectrum disorders. Prog Mol Biol Transl Sci 173:35–60
Chen Y et al (2010) Murine hippocampal neurons expressing Fmr1 gene premutations show early developmental deficits and late degeneration. Hum Mol Genet 19(1):196–208
Wheeler AC et al (2016) Developmental profiles of infants with an FMR1 premutation. J Neurodev Disord 8:40
Gallego PK, Burris JL, Rivera SM (2014) Visual motion processing deficits in infants with the fragile X premutation. J Neurodev Disord 6(1):29
Nolin SL et al. Deficits in Prenatal Serine Biosynthesis Underlie the Mitochondrial Dysfunction Associated with the Autism-Linked FMR1 Gene. Int J Mol Sci, 2021. 22(11).
Girirajan S, et al. (2010) A recurrent 16p12.1 microdeletion supports a two-hit model for severe developmental delay. Nat Genet, 42(3): 203–9.
Kumar RA, et al. (2008) Recurrent 16p11.2 microdeletions in autism. Hum Mol Genet, 17(4): 628–38.
Weiss LA, et al. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med, 2008. 358(7): 667–75.
Hannes FD, et al. (2009) Recurrent reciprocal deletions and duplications of 16p13.11: the deletion is a risk factor for MR/MCA while the duplication may be a rare benign variant. J Med Genet, 46(4): p. 223–32.
Ullmann R et al. (2007) Array CGH identifies reciprocal 16p13.1 duplications and deletions that predispose to autism and/or mental retardation. Hum Mutat. 28(7): 674–82.
Itsara A et al (2009) Population analysis of large copy number variants and hotspots of human genetic disease. Am J Hum Genet 84(2):148–161
Lozano R et al (2014) Genomic studies in fragile X premutation carriers. J Neurodev Disord 6(1):27
Conde V et al (2013) Abnormal GABA-mediated and cerebellar inhibition in women with the fragile X premutation. J Neurophysiol 109(5):1315–1322
Bernard PB et al (2013) Phosphorylation of FMRP and alterations of FMRP complex underlie enhanced mLTD in adult rats triggered by early life seizures. Neurobiol Dis 59:1–17
Acknowledgements
We thank the families who have participated in our studies and also the fragile X team including students at the MIND Institute for their excellent collaboration.
Funding
This research was supported by grants from NICHD including HD036071 and the MIND Institute Intellectual and Developmental Disabilities Research Center P50 HD103526. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author information
Authors and Affiliations
Contributions
RA conceptualized the article, wrote the first draft and subsequent versions of the manuscript. DP was a major contributor to the manuscript and revised versions of the manuscript. RA and DP contributed equally to this paper. RH conceptualized the article and critically reviewed and revised versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
RA and DP have no competing interests pertinent to this publication. RH has received funding from Azrieli Foundation for treatment studies in fragile X syndrome and she has consulted with Zynerba about treatment in fragile X syndrome.
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aishworiya, R., Protic, D. & Hagerman, R. Autism spectrum disorder in the fragile X premutation state: possible mechanisms and implications. J Neurol 269, 4676–4683 (2022). https://doi.org/10.1007/s00415-022-11209-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11209-5