Abstract
Objective
The current study intended to determine the incidence, severity and reversibility of paclitaxel plus carboplatin (CP)–induced peripheral neuropathy (CPPN) and to describe its clinical and electrophysiological features.
Patients and methods
We prospectively studied 21 adult patients scheduled to be treated with 6 courses of cumulative carboplatin plus paclitaxel (CP) regimens for a non–myeloid malignancy. They were followed–up by neurological examination and electrophysiological study during chemotherapy and 3 months after its discontinuation. The severity of neurotoxicity was assessed by means of a modified peripheral neuropathy (PNP) score.
Results
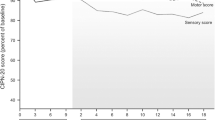
Evidence of CPPN was recorded in 14 of the 21 patients (66.6 %). The sensory symptoms were present in the lower limbs first and then involved the upper limbs. No statistical significance, concerning the changes from baseline to subsequent mean scores in all motor conduction parameters examined,was revealed. By contrast, comparisons of the mean changes at baseline and each of the follow–up studies showed significant decrease in all sensory action potentials examined. The mean PNP scores for patients that manifested some grade of neurotoxicity were 17.9 ± 9.8. The followup data 3 months after the discontinuation of chemotherapy showed that the CP–induced neuropathy was at least partially reversed.
Conclusions
CP–induced neuropathy was symmetrical, distal and predominately sensory in character, though minor to moderate motor signs were only evident in severely affected patients. Reversibility of CPinduced neuropathy was partially observed after the suspension of chemotherapy.
Similar content being viewed by others
References
Argyriou AA, Chroni E, Koutras A, et al. (2005) Vitamin E for prophylaxis against chemotherapy–induced neuropathy: a randomized controlled trial. Neurology 64(1):26–31
Argyriou AA, Polychronopoulos P, Koutras A, et al. (2005) Peripheral neuropathy induced by administration of cisplatin and paclitaxel–based chemotherapy. Could it be predicted? Support Care Cancer 13(8):647–651
Berger T, Malayeri R, Doppelbauer G, et al. (1997) Neurological monitoring of neurotoxicity induced by paclitaxel/ cisplatin chemotherapy. Eur J Cancer 33(9):1393–1399
Chaudhry V, Rowinsky EK, Sartorius SE, et al. (1994) Peripheral neuropathy from taxol and cisplatin combination chemotherapy: Clinical and electrophysiological studies. Ann Neurol 35:304–311
Davidson NG (1996) Single–agent paclitaxel as first–line treatment of metastatic breast cancer: the British experience. Semin Oncol 23(suppl 11):6–10
Dustin P (1980) Microtubules. Sci Am 243:66–76
du Bois A, Luck HJ, Meier W, et al. (2003) Arbeitsgemeinschaft Gynakologische Onkologie Ovarian Cancer Study Group. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/ paclitaxel as first–line treatment of ovarian cancer. J Natl Cancer Inst 95(17):1320–1329
Dyck PJ, Thomas PK (1993) (eds): Peripheral Neuropathy (ed 3). Vol 2. Philadelphia, PA, WB Saunders, pp 1310–1317
Fountzilas G, Kalofonos HP, Dafni U, et al. (2004) Paclitaxel and epirubicin versus paclitaxel and carboplatin as first–line chemotherapy in patients with advanced breast cancer: a phase III study conducted by the Hellenic Cooperative Oncology Group. Ann Oncol 15(10):1517–1526
Gore M, Mainwaring P, A’Hern R, et al. (1998) Randomized trial of dose–intensity with single–agent carboplatin in patients with epithelial ovarian cancer. London Gynaecological Oncology Group. J Clin Oncol 16(7):2426–2434
Huizing MT, van Warmerdam LJ, Rosing H, et al. (1997) Phase I and pharmacologic study of the combination paclitaxel and carboplatin as first–line chemotherapy in stage III and IV ovarian cancer. J Clin Oncol 15(5):1953–1964
Kimura J (2001) Electrodiagnosis in diseases of nerve and muscle, principles and practice (ed 3). Oxford University press, pp 91–166
Kleyweg RP, Van der Meché FGA, Schmitz PIM (1991) Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain–Barré syndrome. Muscle Nerve 14:1103–1109
Lilenbaum RC, Chen CS, Chidiac T, et al. (2005) Phase II randomized trial of vinorelbine and gemcitabine versus carboplatin and paclitaxel in advanced non–small–cell lung cancer. Ann Oncol 16(1):97–101
Markman M, Kennedy A, Webster K, et al. (2001) Neurotoxicity associated with a regimen of carboplatin (AUC 5–6) and paclitaxel (175 mg/m2 over 3 h) employed in the treatment of gynecologic malignancies. J Cancer Res Clin Oncol 127(1):55–58
McGuire WP (1997) How many more nails to seal the coffin of dose intensity? Ann Oncol 8(4):311–313
Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47:207–214
Ozols RF, Bundy BN, Greer BE, et al. (2003) Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol 21(17):3194–3200
Panayiotopoulos CP, Chroni E (1996) F–waves in clinical neurophysiology: a review. Methodological issues and overall value in peripheral neuropathies. Electroencephalogr Clin Neurophysiol 101:365–374
Peltier AC, Russell JW (2002) Recent advances in drug–induced neuropathies. Curr Opin Neurol 15(5):633–638
Quasthoff S, Hartung HP (2002) Chemotherapy–induced peripheral neuropathy. J Neurol 249(1):9–17
Rowinsky EK, Cazenave LA, Donehower RC (1990) Taxol: a novel investigational antimicrotubule agent. J Natl Cancer Inst 82:1247–1259
Rowinsky EK, Eisenhauer EA, Chaudhry V, et al. (1993) Clinical toxicities encountered by paclitaxel (Taxol). Semin Oncol 20:1–15
Rowinsky EK, Donehower RC (1995) Paclitaxel (Taxol). NEJM 332:1004–1014
Schaumburg HH, Spencer PS (1979) Toxic neuropathies. Neurology 29:429–431
Thigpen JT (1997) Dose–intensity in ovarian carcinoma: hold, enough? J Clin Oncol 15(4):1291–1293
Windebank AJ (1999) Chemotherapeutic neuropathy. Curr Opin Neurol 12:565–571
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Argyriou, A.A., Polychronopoulos, P., Iconomou, G. et al. Paclitaxel plus carboplatin–induced peripheral neuropathy. J Neurol 252, 1459–1464 (2005). https://doi.org/10.1007/s00415-005-0887-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-005-0887-8