Abstract
Transgenic (TG) Ca2+/calmodulin-dependent protein kinase II (CaMKII) δC mice develop systolic heart failure (HF). CaMKII regulates intracellular Ca2+ handling proteins as well as sarcolemmal Na+ channels. We hypothesized that CaMKII also contributes to diastolic dysfunction and arrhythmias via augmentation of the late Na+ current (late I Na) in early HF (8-week-old TG mice). Echocardiography revealed severe diastolic dysfunction in addition to decreased systolic ejection fraction. Premature arrhythmogenic contractions (PACs) in isolated isometrically twitching papillary muscles only occurred in TG preparations (5 vs. 0, P < 0.05) which could be completely terminated when treated with the late I Na inhibitor ranolazine (Ran, 5 μmol/L). Force–frequency relationships revealed significantly reduced twitch force amplitudes in TG papillary muscles. Most importantly, diastolic tension increased with raising frequencies to a greater extent in TG papillary muscles compared to WT specimen (at 10 Hz: 3.7 ± 0.4 vs. 2.5 ± 0.3 mN/mm2; P < 0.05). Addition of Ran improved diastolic dysfunction to 2.1 ± 0.2 mN/mm2 (at 10 Hz; P < 0.05) without negative inotropic effects. Mechanistically, the late I Na was markedly elevated in myocytes isolated from TG mice and could be completely reversed by Ran. In conclusion, our results show for the first time that TG CaMKIIδC overexpression induces diastolic dysfunction and arrhythmogenic triggers possibly via an enhanced late I Na. Inhibition of elevated late I Na had beneficial effects on arrhythmias as well as diastolic function in papillary muscles from CaMKIIδC TG mice. Thus, late I Na inhibition appears to be a promising option for diastolic dysfunction and arrhythmias in HF where CaMKII is found to be increased.
Similar content being viewed by others
Introduction
Systolic contractile dysfunction in heart failure (HF) is caused by altered intracellular ion homeostasis and structural remodeling. Several pathomechanisms have been identified. The sarcoplasmic reticulum (SR) Ca2+-ATPase (SERCA) protein levels and its activity are reduced in parallel to a diminished SR Ca2+-uptake capacity in the failing heart [8, 9, 19, 22]. The functional consequence is an impaired SR Ca2+-loading leading to smaller intracellular Ca2+ transients and elevated diastolic Ca2+ levels [3]. Ca2+ homeostasis is further regulated by phosphorylation of several key proteins thereby controlling Ca2+- as well as Na+-fluxes in hypertrophy [13] and HF [15]. In this context, the Ca2+/calmodulin-dependent protein kinase II (CaMKII) is of major importance [15]. The predominant cardiac isoform is CaMKIIδ with the splice variant δC being primarily cytosolic [32]. CaMKII is a serine/threonine protein kinase that modulates several Ca2+-dependent proteins, such as SR Ca2+-release channels (ryanodine receptors, RyR2), phospholamban (PLB) which regulates SERCA and L-type Ca2+-channels.
We and others have recently shown that CaMKIIδ also regulates Na+ channels in myocytes, most likely by association with and phosphorylation of sarcolemmal Na+ channels [1, 31]. Overexpression of CaMKIIδ was associated with an enhanced late I Na that inactivates with much slower kinetics [31]. Although the amplitude of this current is very small compared to peak I Na, the slow inactivation kinetic results in a substantial Na+ entry during the action potential (AP) leading to a significant prolongation of the AP duration [7, 24].
There is strong evidence for an increased late I Na in ventricular myocytes and its contribution to HF via [Na+] i elevation [5, 18, 20, 29]. Mechanistically, augmentation of the late I Na plays a crucial role in impaired contractility and repolarization of the failing myocardium [17, 29]. We have recently shown that an increased late I Na can substantially elevate intracellular Na+ and consequently diastolic Ca2+ via reverse mode of the Na+/Ca2+-exchanger (NCX) leading to diastolic dysfunction in isolated human ventricular end-stage failing myocardium [24].
Furthermore, it is well accepted that an increased late I Na is known to produce arrhythmogenic triggers, such as early afterdepolarizations (EADs) via prolongation of the cardiac AP or delayed afterdepolarizations (DADs) caused by a Na+-dependent diastolic Ca2+ overload and oscillatory SR Ca2+-release which may activate a transient inward current (I Ti) [14, 17, 23].
We have recently shown that pharmacological inhibition of CaMKII reduces cellular proarrhythmogenic events and decreases arrhythmias in vivo in TG CaMKIIδC mice with severe HF due to reduced spontaneous SR Ca2+ release events (Ca2+ sparks) [21]. We hypothesized that CaMKII-dependent late I Na augmentation is a novel cause for diastolic dysfunction and arrhythmias in CaMKII TG mice with non-ischemic compensated HF.
Methods
CaMKIIδC transgenic mice
CaMKIIδC transgenic (TG) mice were generated using an α-MHC promoter as previously described [33]. We used 8-week-old CaMKIIδC-TG mice and age- and sex-matched WT littermates. All animal studies were approved by the Ethics Committee of the Medical Faculty of the University of Göttingen.
Transthoracic echocardiography
Transthoracic echocardiography was performed using a Vevo2100 (VisualSonics, Toronto, Canada) system with a 30 MHz center frequency transducer. Briefly, animals were anesthetized with 3% isoflurane, and temperature-, respiration-, and ECG-controlled anesthesia was maintained with 1.5% isoflurane. Two-dimensional cine loops with frame rates of >200 frames/s of a long-axis view and a short-axis view at mid-level of the papillary muscles as well as M-mode loops of the short-axis view were recorded. Thicknesses of the anterior myocardial wall (AWTh), the posterior myocardial wall (PWTh), the inner diameter of the left ventricle (LVID) and the area of the left ventricular cavity (Area) were measured in systole and diastole from the short-axis view according to standard procedures [4]. Maximal left ventricular length was measured from the long-axis view. Systolic and diastolic left ventricular volumes were calculated using the area–length method. Maximal diastolic radial velocity of the anterior wall was analyzed using the VevoStrain software (VisualSonics, Toronto, Canada). Measurements were obtained by an examiner blinded to the genotype of the animals.
Mouse intact papillary muscle preparation
TG mice were anesthetized using isoflurane. Hearts were rapidly excised and retrogradely perfused with a modified Krebs–Henseleit buffer solution containing (in mmol/L) Na+ 140.5, K+ 5.1, Mg2+ 1.2, Ca2+ 0.25, Cl− 124.9, SO4 2− 1.2, PO4 3− 2.0, HCO3 − 20, glucose 10, and butanedione monoxime (BDM) 20, equilibrated with carbogen (95% O2, 5% CO2, pH 7.4). Intact papillary muscles were isolated from the RV wall using a stereoscopic microscope in a dissection chamber. Cross-sectional dimensions were similar in WT as well as TG mice (width × thickness × π/4, 0.22 ± 0.01 mm2 for TG and 0.16 ± 0.07 mm2 for WT, P = 0.3).
Characterization of contractile phenotype
For isometric force recordings, papillary muscles were mounted in an organ chamber and connected to the force transducer (Scientific Instruments, Heidelberg, Germany). Papillary muscles were superfused with Krebs–Henseleit solution (in mmol/L: NaCl2 116, KCL 5, NaH2PO4 2, MgCl2 1.2, Na2SO4 1.2, NaHCO3 20, CaCl2 initially 0.25; end concentration 1.25, glucose 10) that was also oxygenated with 95% O2 and 5% CO2 (37°C). Isometric contractions were elicited using electrical field stimulation with a basal stimulation frequency of 4 Hz (voltage 25% above threshold, normally 3–5 V, stimulator, Scientific Instruments, Heidelberg, Germany). Ca2+ (0.25 mmol/L) was added every 2 min after a 30 min washout phase until the final concentration of 1.25 mmol/L was reached in order to prevent a Ca2+-induced contracture. After an equilibration period of 20 min, the muscles were gradually stretched until the maximum steady-state twitch force was achieved and 5 μmol/L ranolazine (Ran, Gilead, Palo Alto, USA) or vehicle was added to the bath solution. After having characterized the basal effect of late I Na inhibition, force–frequency relationships were obtained using increasingly higher stimulation rates of 2, 4, 6, 8, 10, and then back to 4 Hz. To measure SR Ca2+-load, post-rest behavior was assessed by using increasing rest intervals of 5 and 10 s between beats at a basal stimulation frequency of 4 Hz [24, 26]. Short periods of rest increase force of contraction of the first beat upon restimulation which is considered to be dependent on SR Ca2+-uptake and release [25]. In addition, persistent premature arrhythmogenic contractions (PACs) were assessed [26]. PACs had to be stable and to persist continuously over 5 min before the effect of Ran was evaluated [26].
Patch-clamp experiments
Ruptured-patch whole-cell voltage-clamp was used to measure I Na as described previously [24, 26]. Microelectrodes (2–3 MΩ) were filled with (mmol/L) 40 CsCl, 80 Cs-glutamate, 10 NaCl, 0.92 MgCl2, 5 Mg-ATP, 0.3 Li-GTP, 10 HEPES, 0.03 niflumic acid, 0.02 nifedipine, 0.004 strophanthidin, 5 BAPTA (tetracesium salt), 1 5,5′-dibromo BAPTA (tetrapotassium salt), 1.49 CaCl2 (free [Ca2+] i = 100 nmol/L; pH 7.2, CsOH). The bath solution contained (mmol/L) 130 NaCl, 10 tetraethylammonium chloride, 4 CsCl, 1 MgCl2, 10 glucose, 10 HEPES, (pH 7.4, NaOH). Myocytes were mounted on the stage of a microscope (Nikon Eclipse TE2000-U, Düsseldorf, Germany). Fast capacitance which is generated largely by the pipette itself was compensated in cell-attached configuration. Liquid junction potentials (3–6 mV) were corrected. Membrane capacitance was compensated after patch rupture, access resistance was typically <7 MΩ. All recordings were started 5 min after rupture. Signals were filtered with 2.9 and 10 kHz Bessel filters and recorded with an EPC10 amplifier (HEKA Elektronik Dr. Schulze GmbH, Lambrecht/Pfalz, Germany). Myocytes were held at −120 mV and late I Na was elicited using 250 ms depolarizing pulses to −30 mV. Each pulse was preceded by a 5 ms pre-pulse to +50 mV in order to optimize voltage control. The measured currents were normalized to the membrane capacitance. I Na decay (first 200 ms) was fitted using a double exponential function y(t) = A 1 exp (–t/τ1) + A 2 exp (–t/τ2) + y 0. All patch-clamp experiments were conducted at room temperature.
Western blots
Myocardium was homogenized in Tris buffer containing in mmol/L: 20 Tris–HCl, pH 7.4, 200 NaCl, 20 NaF, 1 Na3VO4, 1 DTT, 1% Triton X-100 and complete protease inhibitor cocktail (Roche Diagnostics, Grenzach-Wyhlen, Germany). Protein concentration was determined by BCA assay (Pierce Biotechnology, Rockford, USA). For NCX, denatured cell lysates and tissue homogenates (on ice in 2% beta-mercaptoethanol) were subjected to Western blotting (8% SDS-polyacrylamide gels) using anti-NCX (1:5000; Swant, Bellinzona, Switzerland), anti-GAPDH (1:40,000, Biotrend Chemikalien GmbH, Köln, Germany) as primary and an HRP-conjugated donkey anti-rabbit and sheep anti-mouse IgG (1:10,000; Amersham Biosciences, Freiburg, Germany) as secondary antibody. SERCA blots were performed using denatured cell lysates and tissue homogenates (on ice in 2% beta-mercaptoethanol) which were subjected to Western blotting (8% SDS-polyacrylamide gels) using anti-SERCA2 (1:20,000; Affinity BioReagents, Rockford, USA) and anti-GAPDH (1:40,000, Biotrend Chemikalien GmbH, Köln, Germany) as primary and an sheep anti-mouse IgG (1:10,000; Amersham Biosciences, Freiburg, Germany) as secondary antibody. Chemiluminescent detection was done with Immobilion Western Chemiluminescent HRP Substrate (Millipore, Billerica, USA).
Data analysis and statistics
Force values were normalized to the cross-sectional area of each muscle (width × thickness × π/4) and expressed in mN/mm2. All data are shown as mean ± S.E.M. Student’s t test, 2-way or 1-way repeated measures ANOVA with post hoc tests or Fisher`s exact test were used to test for significance. A two-sided P value of <0.05 was considered significant.
Results
Cardiac phenotype of transgenic CaMKIIδC mice
In CaMKIIδC TG mice the heart weight/body weight ratio was increased by ~40% as compared to WT (Fig. 1a–c, n = 13 vs. 14, P < 0.05) indicating an early stage of hypertrophy (8-week-old mice) since this value can increase to more than twofold in 3-month-old mice [16]. Figure 1d shows original M-mode echocardiographic recordings with ventricular systolic dysfunction in a TG as compared to a WT mouse. TG mice have a reduced fractional area shortening (Fig. 1e) as well as a reduced ejection fraction of 27 ± 1% compared to 50 ± 5% in WT mice (Fig. 1f, n = 5 each, P < 0.05). In order to evaluate diastolic function the relaxation velocity was also determined. TG CaMKIIδC mice had markedly impaired relaxation velocities of 1.0 ± 0.4 cm/s compared to 2.3 ± 0.2 cm/s in WT mice (Fig. 1g, P < 0.05). Therefore, these mice suffer from diastolic in addition to systolic dysfunction.
Cardiac phenotype characteristics. a Heart weight of TG CaMKIIδc mice was increased compared to WT (n = 14 vs. 13, P < 0.05), while b body weight remained unchanged. c Mean values of heart weight/body weight ratio (HW/BW). d Representative echocardiographic recordings of a WT and TG CaMKIIδc mouse. Mean values of e fractional area shortening and f ejection fraction (n = 5 each, P < 0.05). g A reduced relaxation velocity in TG CaMKIIδc mice indicates diastolic dysfunction (P < 0.05)
Changes in Ca2+ handling proteins
We investigated SERCA and NCX protein abundance in WT and CaMKIIδC TG left ventricular myocardium to assess their possible involvement in the alterations of diastolic left ventricular performance. We found that SERCA protein levels were reduced to 74 ± 10% in TG CaMKIIδC mice (Fig. 2a, n = 7 vs. 6 WT, P < 0.05), while NCX protein abundance was unaltered (Fig. 2b). Here, it is important to note that NCX protein levels in 3-month-old TG CaMKIIδC mice, a time where these mice develop overt HF, are dramatically increased [16].
Premature contractions in isolated papillary muscles
While muscles from WT mice did not develop PACs [26] we found PACs in all investigated TG muscles (n = 5; P < 0.05, Fisher′s test, Fig. 3a, b). Addition of vehicle solution did not terminate PACs, whereas these were inhibited by adding 5 μmol/L Ran to the bath solution (Fig. 3c, d, P < 0.05, Fisher′s test). These antiarrhythmic effects of Ran were observed within 2 min of exposure to the drug. Thereafter, the papillary muscles remained stable and generated a regular rhythmic stimulation-dependent response.
Spontaneous premature arrhythmogenic contractions (PACs). a Original tracings showing PACs in a TG CaMKIIδc muscle treated with vehicle control. The red marks indicate regular stimulation pulses (4 Hz). b Mean values of PACs in WT and CaMKIIδc muscles (n = 0 vs. 5, P < 0.05, Fisher′s test). c Addition of 5 μmol/L ranolazine (Ran) reversed the PACs in the same preparation back to a stimulation-dependent rhythm. d All PACs in CaMKIIδc muscles could be reversed by Ran addition (P < 0.05, Fisher′s test)
Basal contractility
In another series of experiments the basal effect of 5 μmol/L Ran was investigated in TG muscles stimulated at 4 Hz. After an incubation period of 15 min twitch force amplitude was 81.1 ± 6.3% (normalized to baseline) in vehicle-treated specimen as compared to 69.3 ± 6.7% (n = 9 each, P = 0.22, data not shown) in the presence of 5 μmol/L Ran. While force amplitude was unchanged we found a reduced diastolic tension in Ran-treated muscles to 87.0 ± 2.8% as compared to vehicle with 99.6 ± 5.5% (P < 0.05, Fig. 4a).
a Basal effects of 5 μmol/L ranolazine (Ran) on diastolic tension of TG CaMKIIδc muscles (n = 9 each, P < 0.05). b Mean values of post-rest behavior (ratio of first twitch after rest normalized to the last one before rest, PR/SS) indicating impaired post-rest behavior in TG CaMKIIδc mice (at 10 s of rest: n = 7 each, P < 0.05). Ran did not cause contractile differences compared to vehicle treated specimen (n = 7 vs. 9)
Post-rest behavior of papillary muscles was determined at rest intervals of 5 and 10 s to biomechanically assess SR Ca2+ content [24, 26]. Figure 4b summarizes these results indicating that SR function is impaired in specimen from TG mice while addition of Ran had no further effect on post-rest behavior. At 10 s of rest the first twitch after rest divided by the last before rest (pr/ss) was 2.7 ± 0.5 in WT muscles and was reduced in specimen from TG mice to 1.6 ± 0.2 (Fig. 4b, n = 7 each, P < 0.05). Ran did not affect post-rest behavior in TG papillary muscle (Fig. 4b).
Contractile response at increasing stimulation frequencies
In order to determine the effect of Ran on contractile function, we obtained force–frequency relationships in muscles stimulated at frequencies up to 10 Hz (Fig. 5a). TG papillary muscles presented with lower baseline force compared to WT and a negative staircase phenomenon (RM-ANOVA P < 0.05, WT vs. TG: n = 10 vs. 11; Fig. 5b). In a second series of experiments, TG papillary muscles were treated with 5 μmol/L Ran. This intervention had no effect on twitch force amplitude in TG muscles (n = 11, RM-ANOVA P = 0.6; Fig. 5b).
Force–frequency relationships. a Original tracings of muscles isolated from WT and CaMKIIδc TG mice without and with 5 μmol/L ranolazine (Ran) at increasing frequencies. While the WT muscle did not develop an increase in diastolic tension, the CaMKIIδc TG muscle exhibit a marked diastolic dysfunction that could be reduced Ran. b Mean data of twitch force amplitude of WT and CaMKIIδc TG muscles with and without Ran (n = 10 vs. 11 vs. 11, WT vs. TG, P < 0.05). c Average values showing increases in diastolic tension in CaMKIIδc TG muscles that could be reduced in the presence of Ran (P < 0.05)
In contrast, the TG muscles developed a constant increase in diastolic tension up to 3.7 ± 0.4 mN/mm2 compared to WT specimen with 2.5 ± 0.2 mN/mm2 (n = 11 vs. 10, RM-ANOVA P < 0.05), which we interpret as an ex vivo presentation of diastolic dysfunction. This effect was more pronounced at higher stimulation frequencies. Ran normalized diastolic tension markedly to 2.3 ± 0.3 mN/mm2 (n = 11, RM-ANOVA P < 0.05). Of note, there was no significant reduction of diastolic tension in WT specimen in the presence of Ran (data not shown).
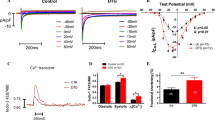
Measurements of the late Na+ current
The slow time constant (τ2) of the double exponential function which largely corresponds to late I Na was prolonged in TG compared to WT myocytes at 0.5 Hz (81.9 ± 8.3 vs. 37.6 ± 3.6 ms, n = 11 vs. 6, P < 0.05, Figs. 6a, b, Table 1). Interestingly, this prolongation of the slow, second inactivation phase in TG could be reversed in the presence of Ran (38.8 ± 4.1 ms, n = 7, P < 0.05 vs. vehicle), which is in accordance with substantial late I Na inhibition. In contrast, there was no significant prolongation of fast I Na decay in TG myocytes vs. WT littermates (Table 1). Moreover, Ran also did not influence fast I Na decay kinetics.
a Original late INa tracings at 1 Hz in WT and CaMKIIδc TG myocytes in the presence and absence of 5 μmol/L ranolazine (Ran). The voltage protocol is shown in the inset. b Na+ current decay was fitted with a double exponential equation. The time constant of the late decay phase was markedly prolonged in myocytes from CaMKIIδc TG mice vs. WT (n = 11 vs. 6, P < 0.05). Addition of Ran shortened this time constant in myocytes from CaMKIIδc TG mice consistent with substantial late INa inhibition (n = 7, P < 0.05)
Discussion
Here, we show for the first time that 8-week-old TG CaMKIIδC mice develop non-ischemic diastolic dysfunction in addition to systolic HF. In these mice, late I Na was markedly higher as compared to WT. Inhibition of late I Na in vitro using Ran led to a significant improvement of diastolic function without negative inotropic effects. Arrhythmogenic events can be found in multicellular preparations of TG CaMKIIδC mice even in the absence of isoprenaline. Moreover, Ran shows antiarrhythmic effects even in the absence of NCX upregulation. In summary, diastolic dysfunction and arrhythmias share a similar molecular mechanism by means of increased diastolic Na+. Both clinical problems may be approached by inhibiting late I Na.
Cardiac overexpression of CaMKIIδC
CaMKIIδC overexpression was shown to be associated with HF [32] and arrhythmias in vivo [21, 31]. CaMKII activity is increased by ~3-fold in TG CaMKIIδC mice [33] which is similar to the increased CaMKII activity observed in failing human hearts [11, 12].
To reduce complex remodeling caused by advanced HF we investigated younger mice (8 weeks old); this is in clear contrast to earlier studies [21, 27, 31]. Interestingly, although these mice are younger compared to those of other reports, the systolic contractile function of the myocardium is already severely depressed as evident by echocardiography. Muscle experiments in vitro revealed impaired contractile force and post-rest behavior. We found a decreased expression of SERCA protein as a typical change that occurs in the failing myocardium contributing to decreased SR Ca2+ content and SR Ca2+ release. Therefore, the TG CaMKIIδC mouse can be regarded as a model of elevated CaMKII activity, but also as a pathophysiological relevant model of non-ischemic HF.
CaMKIIδC, late I Na, and arrhythmias
In the present work, PACs only occurred in papillary muscles isolated from TG CaMKIIδC mice, whereas no arrhythmias were observed in WT specimens. These results are generally in line with our previous findings showing that isoprenaline-induced EADs and DADs are associated with CaMKIIδC overexpression [21]. Inhibition of CaMKII in the previous report depressed EADs and DADs. Moreover, the current data add important information because even without addition of isoprenaline arrhythmogenic events can be found in muscles in vitro from CaMKIIδC TG mice.
It was suggested previously that the SR Ca2+-leak would play an important role in this pathology [21]. Indeed, cardiomyocytes isolated from CaMKIIδC mice have an increased open probability of the RyR leading to increased SR Ca2+-sparks and Ca2+-oscillation which may trigger I Ti and arrhythmias [16, 21, 31].
However, in addition to SR Ca2+ leak, prolonged APs [17] are also observed in these mice which may increase the propensity for EADs. Late I Na is known to prolong the AP and thereby likely contribute to EADs [23]. Since CaMKII was shown to regulate Na+ channels, it is possible that this mechanism is also crucially involved in the arrhythmogenesis observed. Hence, antiarrhythmic effects of CaMKII inhibition may actually be mediated largely through late I Na.
Therefore, we tested whether inhibition of late I Na using Ran would lead to a similar reduction of the arrhythmic propensity as described previously [22]. Ran has been shown to selectively inhibit late I Na vs. peak I Na (especially at concentrations of 5 μmol/L) [2] and we now show indeed that Ran reduces late I Na in TG myocytes. This is in contrast to another study where CaMKII inhibition could not reduce intracellular Na+ [32] (through late I Na) and may be explained by the fact that in the current report young mice where used in which only a functional upregulation of late I Na may occur. In contrast in older mice, several other mechanisms may be involved, such as altered expression/trafficking of Na+ channels, involvement of other kinases activated during congestive HF, as well as less altered local phosphatase binding/expression at the Na+ channel (as reported for RyR).
Nevertheless, we found a strong effect of Ran on PACs in CaMKIIδC specimen. This effect commonly occurred within minutes and kept the preparation stable in a stimulation-dependent rhythm. These results also indicate that the late I Na may play a crucial role in the arrhythmogenesis in failing hearts and that blockade of late I Na appears useful to control HF associated arrhythmias.
Inhibition of late I Na ameliorates diastolic dysfunction
Elevated diastolic Ca2+ as a consequence of reverse NCX activity in Na+-overloaded cardiomyocytes may on the one hand cause arrhythmias and on the other hand also impair diastolic contractile performance of the heart. Echocardiographic analyses confirmed diastolic dysfunction in TG CaMKIIδC mice. Moreover, in isometrically twitching preparations we found a frequency-dependent increase in diastolic tension compared to WT specimen which may be an additional indication for increased diastolic Ca2+. This may be at least partly explained by the finding that SERCA protein is downregulated while NCX is still unchanged in TG hearts isolated from 8-week-old mice, whereas older mice (e.g. 3 months) exhibit increased NCX expression levels [16]. Another possibility for elevated diastolic Ca2+ levels (and thus altered mechanical function) may be a slight albeit not significant downregulation of calsequestrin protein expression (data not shown). Calsequestrin is a low-affinity, high-capacity Ca2+-binding protein in the SR which is often used as a housekeeping gene. Therefore, we preferred to normalize our Western blot results to GAPDH which is unaltered. Nevertheless, we acknowledge that even slight alterations of calsequestrin expression and hence impaired SR Ca2+ storage capacity might be a limitation with respect to the interpretation of our study but the calsequestrin downregulation does not reach the extent as shown in another study with a clear and significant 20% downregulation (vs. WT) when CaMKIIδc transgenic mice were crossbred with RyR2R4496C mutated mice [6]. It is important to note that to date neither multicellular tissue nor cardiomyocytes isolated from TG CaMKIIδC mice have been investigated at physiological heart rates (up to 10 Hz). Here, we show that diastolic tension increased at 6 Hz and above.
Wagner et al. have previously shown that overexpression of CaMKIIδC results in an increase of [Na] i that could be significantly reduced by CaMKII-inhibitors in acute CaMKIIδC overexpressing myocytes [31]. However, a similar increase of [Na] i could not be reversed by CaMKII inhibition in TG CaMKIIδC mice at 3 months of age suggesting that there may be other sources of Na+ entry or CaMKII-independent mechanism involved that may have to do with the remodeling processes during HF progression. However, since late I Na inhibition can shorten the AP, a reduced reverse mode NCX activity may be the consequence leading to less Ca2+ overload and less diastolic dysfunction.
In the present work, we were interested whether inhibition of elevated late I Na in TG myocytes might improve contractile function. Indeed, we found evidence for a reduced diastolic dysfunction in the presence of Ran. This effect could be observed under several conditions. Interestingly, this effect was not paralleled by any negative inotropy. The findings of our study are in agreement with our previous report showing improved diastolic function due to inhibition of late I Na [24, 26, 28]. Most importantly, in isolated human failing myocardium CaMKII and late I Na are markedly elevated [11, 12, 18, 31].
Our results indicate that the late I Na plays a crucial role in determining diastolic function. Inhibition of late I Na may counteract the CaMKII effect that induces elevation of this current leading to Na+ overload of the cell. Moreover, the fact that the Ran effect was pronounced at higher frequencies supports that concept that AP shortening may be an additional mechanism of Ran-dependent improvement of diastolic function. Since diastolic dysfunction can be induced by structural as well as electrical remodeling [10], the sensitivity of TG myocardium to Ran and the acute effect observed in our experiments shows that diastolic function is strongly related to electrical remodeling. However, there are also types of HF showing diastolic dysfunction which depends mainly on fibrosis [30].
Conclusions
Chronic overexpression of CaMKIIδC leads to diastolic and systolic dysfunction as well as an increased propensity for arrhythmias. Inhibition of an increased late I Na in TG myocardium was effective in treating diastolic dysfunction and arrhythmias. Hence the relation between elevated CaMKII activity and increased late I Na in HF may be of therapeutic interest and merits further investigation.
References
Aiba T, Hesketh GG, Liu T, Carlisle R, Villa-Abrille MC, O’Rourke B, Akar FG, Tomaselli GF (2010) Na+ channel regulation by Ca2+/calmodulin and Ca2+/calmodulin-dependent protein kinase II in guinea-pig ventricular myocytes. Cardiovasc Res 85:454–463. doi:10.1093/cvr/cvp324
Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Thomas G (2004) Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation 110:904–910. doi:10.1161/01.CIR.0000139333.83620.5D
Beuckelmann DJ, Nabauer M, Erdmann E (1992) Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation 85:1046–1055
Collins KA, Korcarz CE, Lang RM (2003) Use of echocardiography for the phenotypic assessment of genetically altered mice. Physiol Genomics 13:227–239. doi:10.1152/physiolgenomics.00005.2003
Despa S, Islam MA, Weber CR, Pogwizd SM, Bers DM (2002) Intracellular Na+ concentration is elevated in heart failure but Na+/K+ pump function is unchanged. Circulation 105:2543–2548. doi:10.1161/01.CIR.0000016701.85760.97
Dybkova N, Sedej S, Napolitano C, Neef S, Rokita AG, Hünlich M, Heller Brown J, Kockskämper J, Priori SG, Pieske B, Maier LS. Overexpression of CaMKIIδc in RyR2R4496C knock-in mice leads to spontaneous sudden cardiac death and altered intracellular Ca2+ handling. J Am Coll Cardiol (in press) doi: 10.1016/j.jacc.2010.08.639
Hale SL, Shryock JC, Belardinelli L, Sweeney M, Kloner RA (2008) Late sodium current inhibition as a new cardioprotective approach. J Mol Cell Cardiol 44:954–967. doi:10.1161/01.CIR.0000016701.85760.97
Hasenfuss G, Reinecke H, Studer R, Meyer M, Pieske B, Holtz J, Holubarsch C, Posival H, Just H, Drexler H (1994) Relation between myocardial function and expression of sarcoplasmic reticulum Ca2+-ATPase in failing and nonfailing human myocardium. Circ Res 75:434–442
Hasenfuss G, Schillinger W, Lehnart SE, Preuss M, Pieske B, Maier LS, Prestle J, Minami K, Just H (1999) Relationship between Na+–Ca2+-exchanger protein levels and diastolic function of failing human myocardium. Circulation 99:641–648
Heusch G (2009) Diastolic heart failure: a misNOmer. Basic Res Cardiol 104:465–467. doi:10.1007/s00395-009-0025-3
Hoch B, Meyer R, Hetzer R, Krause EG, Karczewski P, MaxDelbrèuck Center for Molecular Medicine B-BG (1999) Identification and expression of delta-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ Res 84:713–721
Kirchhefer U, Schmitz W, Scholz H, Neumann J (1999) Activity of cAMP-dependent protein kinase and Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human hearts. Cardiovasc Res 42:254–261. doi:10.1016/S0008-6363(98)00296-X
Klaiber M, Kruse M, Volker K, Schroter J, Feil R, Freichel M, Gerling A, Feil S, Dietrich A, Londono JE, Baba HA, Abramowitz J, Birnbaumer L, Penninger JM, Pongs O, Kuhn M (2010) Novel insights into the mechanisms mediating the local antihypertrophic effects of cardiac atrial natriuretic peptide: role of cGMP-dependent protein kinase and RGS2. Basic Res Cardiol 105:583–595. doi:10.1007/s00395-010-0098-z
Lederer WJ, Tsien RW (1976) Transient inward current underlying arrhythmogenic effects of cardiotonic steroids in Purkinje fibres. J Physiol 263:73–100
Maier LS, Bers DM (2007) Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc Res 73:631–640. doi:10.1016/j.cardiores.2006.11.005
Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM (2003) Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res 92:904–911. doi:10.1161/01.RES.0000069685.20258.F1
Maltsev VA, Sabbah HN, Higgins RS, Silverman N, Lesch M, Undrovinas AI (1998) Novel, ultraslow inactivating sodium current in human ventricular cardiomyocytes. Circulation 98:2545–2552
Maltsev VA, Silverman N, Sabbah HN, Undrovinas AI (2007) Chronic heart failure slows late sodium current in human and canine ventricular myocytes: implications for repolarization variability. Eur J Heart Fail 9:219–227. doi:10.1016/j.ejheart.2006.08.007
Meyer M, Schillinger W, Pieske B, Holubarsch C, Heilmann C, Posival H, Kuwajima G, Mikoshiba K, Just H, Hasenfuss G et al (1995) Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation 92:778–784
Pieske B, Maier LS, Piacentino V 3rd, Weisser J, Hasenfuss G, Houser S (2002) Rate dependence of [Na+]i and contractility in nonfailing and failing human myocardium. Circulation 106:447–453. doi:10.1161/01.CIR.0000023042.50192.F4
Sag CM, Wadsack DP, Khabbazzadeh S, Abesser M, Grefe C, Neumann K, Opiela MK, Backs J, Olson EN, Brown JH, Neef S, Maier SK, Maier LS (2009) Calcium/calmodulin-dependent protein kinase II contributes to cardiac arrhythmogenesis in heart failure. Circ Heart Fail 2:664–675. doi:10.1161/CIRCHEARTFAILURE.109.865279
Schwinger RH, Bohm M, Schmidt U, Karczewski P, Bavendiek U, Flesch M, Krause EG, Erdmann E (1995) Unchanged protein levels of SERCA II and phospholamban but reduced Ca2+ uptake and Ca2+-ATPase activity of cardiac sarcoplasmic reticulum from dilated cardiomyopathy patients compared with patients with nonfailing hearts. Circulation 92:3220–3228
Song Y, Shryock JC, Belardinelli L (2008) An increase of late sodium current induces delayed afterdepolarizations and sustained triggered activity in atrial myocytes. Am J Physiol Heart Circ Physiol 294:H2031–H2039. doi:10.1152/ajpheart.01357.2007
Sossalla S, Wagner S, Rasenack EC, Ruff H, Weber SL, Schondube FA, Tirilomis T, Tenderich G, Hasenfuss G, Belardinelli L, Maier LS (2008) Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts—role of late sodium current and intracellular ion accumulation. J Mol Cell Cardiol 45:32–43. doi:10.1016/j.yjmcc.2008.03.006
Sossalla S, Fluschnik N, Schotola H, Ort KR, Neef S, Schulte T, Wittköper K, Renner A, Schmitto JD, Gummert J, El-Armouche A, Hasenfuss G, Maier LS (2010) Inhibition of elevated Ca2+/calmodulin-dependent protein kinase II (CaMKII) improves contractility in human failing myocardium. Circ Res 107:1150–1161. doi:10.1161/CIRCRESAHA.110.220418
Sossalla S, Kallmeyer B, Wagner S, Mazur M, Maurer U, Toischer K, Schmitto JD, Seipelt R, Schondube FA, Hasenfuss G, Belardinelli L, Maier LS (2010) Altered Na+ currents in atrial fibrillation effects of ranolazine on arrhythmias and contractility in human atrial myocardium. J Am Coll Cardiol 55:2330–2342. doi:10.1016/j.jacc.2009.12.055
Studer R, Reinecke H, Bilger J, Eschenhagen T, Bohm M, Hasenfuss G, Just H, Holtz J, Drexler H (1994) Gene expression of the cardiac Na+–Ca2+ exchanger in end-stage human heart failure. Circ Res 75:443–453
Undrovinas AI, Belardinelli L, Undrovinas NA, Sabbah HN (2006) Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol 17(Suppl 1):S169–S177. doi:10.1111/j.1540-8167.2006.00401.x
Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, Kamp TJ, Makielski JC (2005) Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol 38:475–483. doi:10.1016/j.yjmcc.2004.12.012
Villari B, Sossalla S, Ciampi Q, Petruzziello B, Turina J, Schneider J, Turina M, Hess OM (2009) Persistent diastolic dysfunction late after valve replacement in severe aortic regurgitation. Circulation 120:2386–2392. doi:10.1161/CIRCULATIONAHA.108.812685
Wagner S, Dybkova N, Rasenack ECL, Jacobshagen C, Fabritz L, Kirchhof P, Maier SKG, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS (2006) Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest 116:3127–3138. doi:10.1172/JCI26620
Zhang T, Brown JH (2004) Role of Ca2+/calmodulin-dependent protein kinase II in cardiac hypertrophy and heart failure. Cardiovasc Res 63:476–486. doi:10.1016/j.cardiores.2004.04.026
Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J Jr, Bers DM, Brown JH (2003) The delta C isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res 92:912–919. doi:10.1161/01.RES.0000069686.31472.C5
Acknowledgments
We gratefully acknowledge the expert assistance of M. Gelo, T. Schulte and T. Sowa. Dr. Sossalla is funded by the Research Program, Faculty of Medicine, Georg-August-University Göttingen. Dr. Maier is funded by the Deutsche Forschungsgemeinschaft (DFG) through the Clinical Research group KFO155 (MA 1982/2-2) and a Heisenberg grant (MA 1982/4-1), as well as by the Fondation Leducq Award to the Alliance for CaMK Signaling in Heart Disease. Dr. Zimmermann is funded by the DFG (ZI 708/7-1,/8-1,10-1) and the Bundesministerium für Bildung und Forschung (BMBF).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Sossalla, S., Maurer, U., Schotola, H. et al. Diastolic dysfunction and arrhythmias caused by overexpression of CaMKIIδC can be reversed by inhibition of late Na+ current. Basic Res Cardiol 106, 263–272 (2011). https://doi.org/10.1007/s00395-010-0136-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-010-0136-x