Summary
Background: The best nutritional option for newborn infants is mother's milk. However, some newborn babies may not be exlusively breastfed during the first months of life, potentially leading to reduced overall health status and the early onset of allergic diseases in some infants. Considerable research has been devoted to the development and assessment of infant nutrition programmes, particularly to the prevention of allergies in high-risk infants. However, equal numbers of infants with and without an elevated familial risk of allergies will eventually develop allergic diseases. Therefore, optimizing nutritional programmes for the early infant population as a whole is an important – but as yet insufficiently studied – area of investigation. Moreover, although safe and effective nutrition must primarily support healthy development of the infant, few studies have evaluated the overall health benefits of nutritional interventions, but have focussed on specific allergic manifestations. In animal models, an allergen-reduced moderate whey hydrolysate formula (pHF, Nestlé Beba HA) induces the development of oral tolerance towards cow's milk proteins, without inducing sensitization. In infants with a high risk for allergies, pHF formulae reduce the early onset of allergic disease during the first 5 years of life by approximately 50% compared with a dietary regimen of unaltered proteins. At present, very little is known about the overall health benefits of such a dietary intervention on the unselected infant population as a whole.
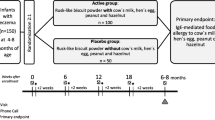
Aim of the study: The aim of our prospective, controlled study was to investigate the overall health benefits of an allergen-reduced nutritional programme in a newborn infant population unselected for atopic risk factors. The population in our study was a comparable as possible to the general population of healthy newborn infants. Our study included exclusive breastfeeding, use of a moderate whey hydrolysate formula (pHF, Nestlé Beba HA) if infant formula was needed, and delayed introduction of low-allergenic weaning foods. The study included assessments of compliance with the dietary programme, and evaluated nutritional habits, growth, and overall health status for 24 months. The health evaluation included allergic manifestations but die – by purpose – not define or evaluate them specifically. Part I of this paper gives results for nutritional habits during the first 6 months of life, Part II gives results for growth and general health status for the same time period, Part III will present feeding habits during the second half of the first year of life, and Part IV will present results to 24 months of age. The complete study report is published as a supplement to this journal.
Methods: Nuritional assignment was to demographically comparable intervention (Z) or control (FF) cohorts according to the infant's place of birth. In the intervention cohort (Z, n=564), the recommended dietary regimen was breastfeeding and/or the pHF formula, with no weaning food before 4 months of age. In the control cohort (FF, n=566), there was no intervention. Longitudinal diet groups, defined for 4 months, excluding dropouts and noncompliants, were exclusive breastfeeding (eBF, Z, n=201, FF, n=162), partial breastfeeding (pBF, Z, n=222, FF, n=311), or non-breastfeeding (nBF, Z, n=42, FF, n=62). Imbalances between groups and cohorts in confounding factors that could influence health-related symptoms were integrated as covariates into the main analyses using logistic regression. Nutritional surveillance was carried out using continuous prospective monitoring.
Results: The overall rate of breastfeeding, irrespective of partial or excluive breastfeeding or the additional use of weaning foods, was similar in both cohorts at 4 and 6 months. However, from ages 3 to 6 month, significantly more Z than FF infants were excluively breastfed (p<0.05), and weaning foods were introduced at a significantly later age in Z than FF (22 versus 18 weeks; p<0.0001). Infants in the Z cohort received fewer different kinds of weaning foods compared with FF (2 versus 10; p<0.0001). Six-month rates of dropout were very low in both groups (Z=4.3%, FF=1.8%) and compliance with the programme was excellent over the strict intervention period of 4 months (Z=86.9%) until the sixth month.
Conclusions: A dietary recommendation that promotes breastfeeding, includes a moderate whey hydrolysate formula (Nestlé Beba HA), and delays the introduction of highly allergenic weaning foods (an allergen-reduced dietary regimen) significantly increases breastfeeding and delays weaning in a normal infant population without any compliance problem to the moderate hydroysate formula that is quite often seen in the eHF. The regimen was followed closely, with a high degree of compliance and a low dropout rate compared with previous allergy-prevention studies. Compliance with the dietary regimen was excellent over the 4 months of intervention and throughout the following 2 months.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 14 December 1999, Accepted: 2 May 2000
Rights and permissions
About this article
Cite this article
Exl, BM., Deland, U., Secretin, MC. et al. Improved general health status in an unselected infant population following an allergen reduced dietary intervention programme The ZUFF-STUDY-PROGRAMME Part I: study design and 6-month nutritional behaviour . Eur J Nutr 39, 89–102 (2000). https://doi.org/10.1007/s003940070024
Issue Date:
DOI: https://doi.org/10.1007/s003940070024