Abstract
Background
Intestinal failure-associated liver disease (IFALD) remains a serious problem in the treatment of infants with nutritional problems and short bowel syndrome.
Methods
A review of the recent literature from 2010 to 2016, concentrating on articles related to the pathophysiology of IFALD and to outcomes of novel nutritional and pharmacological therapies for neonatal cholestasis in the post-surgical neonate.
Results
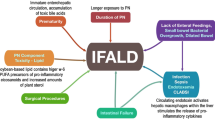
The pathophysiology of IFALD relates to an increase sensitivity of the neonatal liver to cholestasis in the non-fed state; prolonged cholestasis almost inevitably results in liver damage which will progress from fibrosis to cirrhosis. Clinically discerned risk factors include premature birth, inflammation, sepsis, disruption of the enterohepatic circulation by creation of a proximal stoma, and the duration and type of parenteral nutritional support. Within the hepatocyte, the regulatory enzyme farsanoid receptor X (FXR) appears to play a pivotal role in the development of cholestasis. Recent studies have shown that its activity is suppressed by sepsis, and by plant phytosterols found in soy-based lipid preparations. This paradigm is reflected in the emerging consensus for the care of post-surgical neonates, which is based around a multi-disciplinary team approach. Using an algorithm-driven approach, an appropriate balance between caloric support and prevention of IFALD can be achieved.
Conclusions
Further prospective studies are required to further refine the optimal sequence of use of these therapies and the long-term effects on neurological development and hepatic function. However, with optimal care, the number of IF patients progressing to end-stage liver disease because of IFALD should be very low.


Similar content being viewed by others
References
Squires RH, Duggan C, Teitelbaum DH, Wales PW, Balint J, Venick R et al (2012) Natural history of pediatric intestinal failure: initial report from the Pediatric Intestinal Failure Consortium. J Pediatr 161(4):723–728
Kocoshis SA (2010) Medical management of pediatric intestinal failure. Semin Pediatr Surg 19(1):20–26
Sigalet D, Boctor D, Brindle M, Lam V, Robertson M (2011) Elements of successful intestinal rehabilitation. J Pediatr Surg 46(1):150–156
Stanger JD, Oliveira C, Blackmore C, Avitzur Y (2013) Wales PA The impact of multi-disciplinary intestinal rehabilitation programs on the outcome of pediatric patients with intestinal failure: a systematic review and meta-analysis. J Pediatr Surg 48:983–992
Lauriti G, Zani A, Aufieri R, Cananzi M, Chiesa PL, Eaton S, Pierro A (2014) Incidence, prevention, and treatment of parenteral nutrition-associated cholestasis and intestinal failure-associated liver disease in infants and children: a systematic review. JPEN J Parenter Enteral Nutr 38(1):70–85. doi:10.1177/0148607113496280
Karpen SJ (2002) Update on the etiologies and management of neonatal cholestasis. Clin Perinat. 29:159–180
Geier A, Mb Wagner, Dietrich CG, Trauner M (2007) Principles of hepatic organic anion transporter regulation during cholestasis, inflammation and liver regeneration. Biochim et Biophys Acta 1773:283–308
Beath SV, Davies P, Papadopolou A et al (1996) Parenteral nutrition-related cholestasis in postsurgical neonates: multivariate analysis of risk factors. J Pediatr Surg 31:604–606
Lacaille F, Gupte G, Colomb V, D’Antiga L, Hartman C, Hojsak I, Kolacek S, Puntis J, Shamir R; ESPGHAN Working Group of Intestinal Failure and Intestinal Transplantation (2015) Intestinal failure-associated liver disease: a position paper of the ESPGHAN Working Group of Intestinal Failure and Intestinal Transplantation. J Pediatr Gastroenterol Nutr 60(2):272–283. doi:10.1097/MPG.0000000000000586
Wagner M, Zollner G (2009) Trauner M New molecular insights into the mechanisms of cholestasis J. Hepatology 51:565–580
Burrin DG, Ng K, Stoll B, Pipaón MSG (2014) Impact of new-generation lipid emulsions on cellular mechanisms of parenteral nutrition-associated liver disease. Adv Nutr 5:82–91. doi:10.3945/an.113.004796
Stoll B, Horst DA, Cui L, Chang X, Ellis KJ, Hadsell DL, Suryawan A, Kurundkar A, Maheshwari A, Davis TA et al (2010) Chronic parenteral nutrition induces hepatic inflammation, steatosis, and insulin resistance in neonatal pigs. J Nutr 140:2193–2200
Kim MS, Shigenaga J, Moser A, Feingold K, Grunfeld C (2003) Repression of farnesoid X receptor during the acute phase response. J Biol Chem 278:8988–8995
Carter BA, Taylor OA, Prendergast DR, Zimmerman TL, Von Furstenberg R, Moore DD, Karpen SJ (2007) Stigmasterol, a soy lipid-derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR. Pediatr Res 62:301–306
Forchielli ML, Bersani G, Tala S et al (2010) The spectrum of plant and animal sterols in different oil-derived intravenous emulsions. Lipids 45:63. doi:10.1007/s11745-009-3371
Karim CEK, Anderson AL, Devereaux MW et al (2013) Phytosterols promote liver injury and kupffer cell activation in parenteral nutrition-associated liver disease. Sci Transl Med 5(206):206137
Goulet O, Olieman J, Ksiazyk J, Spolidoro J, Tibboe D, Köhler H, Vural Yagci R, Falconer J, Grimble G, Beattie RM (2013) Neonatal short bowel syndrome as a model of intestinal failure: physiological background for enteral feeding. Clinical Nutrition 32:162–171
Cristofalo EA, Schanler RJ, Blanco CL et al (2013) Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr 163:1592–1595
Stamm DA, Hait E, Litman HJ, Mitchell PD, Duggan C (2016) High prevalence of eosinophilic gastrointestinal disease in children with intestinal failure. J Pediatr Gastroenterol Nutr 63(3):336–339
Mazon A, Solera E, Alentado N et al (2008) Frequent IgE sensitization to latex, cow’s milk, and egg in children with short bowel syndrome. Pediatr Allergy Immunol 19:180–183
Javid PJ, Collier S, Richardson D, Iglesias J, Gurac K, Lob C, Kim HB, Duggan C, Tom Jaksic T (2005) The role of enteral nutrition in the reversal of parenteral nutrition-associated liver dysfunction in infants. J Pediatr Surg 40:1015–1018
Teitelbaum DH, Tracy TF Jr, Aouthmany MM, Llanos A, Brown MB, Yu S et al (2005) Use of cholecystokinin-octapeptide for the prevention of parenteral nutrition-associated cholestasis. Pediatrics 115:1332–1340
Heubi JE, Wiechmann DA, Creutzinger V, Setchell KD, Squires R Jr, Couser R et al (2002) Tauroursodeoxycholic acid (TUDCA) in the prevention of total parenteral nutrition-associated liver disease. J Pediatr 141:237–242
Meehan JJ, Georgeson KE (1997) Prevention of liver failure in parenteral nutrition-dependent children with short bowel syndrome. J Pediatr Surg 32:473–475
Ng PC, Lee CH, Wong SPS et al (2007) High-dose oral erythromycin decreased the incidence of parenteral nutrition-associated cholestasis in preterm infants. Gastroenterology 132:1726–1739
Ng YY, Su PH, Chen JY et al (2012) Efficacy of intermediate-dose oral erythromycin on very low birth weight infants with feeding intolerance. Pediatr Neonatol. 53:34–40
Kapoor V, Glover R, Malviya MN (2015) Alternative lipid emulsions versus pure soy oil based lipid emulsions for parenterally fed preterm infants. Cochrane Database Syst Rev. doi:10.1002/14651858.CD009172.pub2
Vanek VW, Seidner DL, Allen P, Bistrian B, Collier S, Gura K, Miles JM, Valentine CJ, Kochevar M, Novel Nutrient Task Force, Intravenous Fat Emulsions Workgroup, American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors (2012) A.S.P.E.N. position paper: clinical role for alternative intravenous fat emulsions. Nutr Clin Pract. 27(2):150–192
Nandivada P, Fell GL, Gura KM, Puder M (2016) Lipid emulsions in the treatment and prevention of parenteral nutrition-associated liver disease in infants and children. Am J Clin Nutr 103(2):629S–634S. doi:10.3945/ajcn.114.103986
Innes S (2009) Omega-3 fatty acids and neural development to 2 years of age: do we know enough for dietary recommendations? J Pediatr Gastroenterol Nutr 48(Supplement):S16–S24
Finn KL, Chung M, Rothpletz-Puglia P (2015) Byham-Gray L impact of providing a combination lipid emulsion compared with a standard soybean oil lipid emulsion in children receiving parenteral nutrition: a systematic review and meta-analysis. JPEN J Parenter Enteral Nutr 39(6):656–667
Lam G, Strogach IG, Baron N, Thompson JF (2016) Normal growth and essential fatty acid status in children with intestinal failure on lipid limitation. J Pediatr Gastroenterol Nutr 62(2):335–340
Diamond IR, Grant RC, Pencharz PB, de Silva N, Feldman BM, Fitzgerald P, Sigalet D, Dicken B, Turner J, Marchand V, Ling SC, Moore AM, Avitzur Y, Wales PW (2016) Preventing the progression of intestinal failure-associated liver disease in infants using a composite lipid emulsion: a pilot randomized controlled trial of SMOFlipid. JPEN J Parenter Enteral Nutr. doi:10.1177/0148607115626921
Rayyan M, Devlieger H, Jochum F, Allegaert K (2012) Short-term use of parenteral nutrition with a lipid emulsion containing a mixture of soybean oil, olive oil, medium-chain triglycerides, and fish oil: a randomized double-blind study in preterm infants. JPEN J Parenter Enteral Nutr 36(1 Suppl):81S–94S PMID: 22237883
Gura KM, Lee S, Valim C, Zhou J, Kim S, Modi BP, Arsenault DA, Strijbosch RAM, Lopes S, Duggan C (2008) Puder M safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics 121:e679–e686
Lam HS, Tam YH, Poon TC, Cheung HM, Yu X, Chan BP, Lee KH, Lee BS, Ng PC (2014) A double-blind randomised controlled trial of fish oil-based versus soy-based lipid preparations in the treatment of infants with parenteral nutrition-associated cholestasis. Neonatology 105:290–296. doi:10.1159/000358267
Park HW, Lee NM, Kim JH, Kim KS, Kim SN (2015) Parenteral fish oil-containing lipid emulsions may reverse parenteral nutrition-associated cholestasis in neonates: a systematic review and meta-analysis. J Nutr 145(2):277–283. doi:10.3945/jn.114.204974
Koletzko B, Goulet O (2010) Fish oil containing intravenous lipid emulsions in parenteral nutrition-associated cholestatic liver disease. Curr Opin Clin Nutr Metab Care 13(3):321–326. doi:10.1097/MCO.0b013e3283385407
Park HW, Lee NM, Kim JH, Kim KS, Kim SN (2014) Parenteral fish oil—containing lipid emulsions may reverse parenteral nutrition–associated cholestasis in neonates: a systematic review and meta-analysis. J Nutr Dis. doi:10.3945/jn.114.204974
Blakely ML, Tyson JE, Lally KP et al (2006) Laparotomy versus peritoneal drainage for necrotizing enterocolitis or isolated intestinal perforation in extremely low birth weight infants: outcomes through 18 months adjusted age. Pediatrics 117(4):e680–e687
Schulzke SM, Deshpande CG, Patole SK (2007) Neurodevelopmental outcomes of very low-birth-weight infants with necrotizing enterocolitis a systematic review of observational studies. Arch Pediatr Adolesc Med 161:583–590
Fitzgibbons SC, Jones BA, Hulla MA, Zurakowskic D, Duro D, Duggan C, Boctor D, Sigalet DL, Jaksic T (2010) Relationship between biopsy-proven parenteral nutrition-associated liver fibrosis and biochemical cholestasis in children with short bowel syndrome. J Ped Surg 45:95–99
Nandivada P, Chang MI, Potemkin AK, Carlson SJ, Cowan E, Oʼloughlin AA, Mitchell PD, Gura KM, Puder M (2015) The natural history of cirrhosis from parenteral nutrition-associated liver disease after resolution of cholestasis with parenteral fish oil therapy. Ann Surg 261(1):172–179. doi:10.1097/SLA.0000000000000445
Acknowledgements
These studies were supported by the Professorship in Pediatric Surgical Research of the Alberta Children’s Hospital Research Foundation and by Sidra Medical and Research Center, Doha Qatar.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Al-Shahwani, N.H., Sigalet, D.L. Pathophysiology, prevention, treatment, and outcomes of intestinal failure-associated liver disease. Pediatr Surg Int 33, 405–411 (2017). https://doi.org/10.1007/s00383-016-4042-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-016-4042-7