Abstract
The diagnosis of myocarditis is difficult and is generally one of exclusion. Moreover, endomyocardial biopsy (EMB) is not a sensitive technique. Magnetic resonance imaging (MRI), however, has shown promising results in diagnosing myocarditis. We evaluated 20 patients with a clinical suspicion of acute myocarditis. Troponin I levels were elevated in 17/20 patients. Cardiac catheterization (n = 13) showed no evidence of coronary artery disease, while normal findings were reported in all five patients who underwent EMB. MRI performed 9.8 ± 7.5 days after the onset of symptoms showed an LV-EDV of 172 ± 50 ml and LV-EF of 57 ± 10%. Abnormalities on delayed contrast-enhanced MRI were found in 15/20 patients, involving 3.7 ± 2.1 segments using the 17-segment model. The lateral LV wall was most frequently involved (61% of enhanced segments). The enhancement was most frequently subepicardial, less often transmural, or midwall (respectively, 67%, 22%, and 11% of enhanced segments). Mild to moderate systolic wall motion abnormalities were invariably found in the abnormally enhancing myocardium on MRI. Associated pericardial effusion was found in six, pericardial enhancement in nine patients. In conclusion, the present study suggests an important role for MRI in evaluating patients with clinical suspicion of acute myocarditis. Not only can the myocardial damage be precisely depicted but also concomitant involvement of the pericardium and impact on regional and global ventricular function can be assessed.
Similar content being viewed by others
Introduction
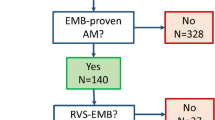
The diagnosis of myocarditis remains difficult. Myocarditis is rarely recognized clinically, mainly because of lack of specific clinical signs, and the diagnosis is generally one of exclusion [1]. Myocarditis is basically an inflammation of the cardiac muscle associated with necrosis of adjacent myocytes but different from those seen after ischemic changes (Dallas criteria for classification) [1]. It is most commonly caused by viral infections, but may also result from hypersensitivity, trauma, radiation, and chemical and physical agents [2]. Endomyocardial biopsy (EMB) is still considered the reference technique for diagnosis of myocarditis. However, there remain many limitations with EMB. For example, in the Myocarditis Treatment Trial assessing 2,233 patients with unexplained heart failure, the EMB criteria for active myocarditis were only met in 9% of patients [3]. Myocarditis usually presents not as a generalized but as a focal or patchy inflammation, often not involving the right ventricle, thus questioning the value of a right ventricular EMB [4]. Moreover, some patients have purely humoral and cytokine mediated forms of myocarditis with little or no cellular infiltrate. Even in postmortem hearts with proven myocarditis, the probability to meet the Dallas criteria with one biopsy is only 17–28% and with more than five biopsies approximately two-thirds of patients met the Dallas criteria [1, 3].
Clearly EMB is not a sensitive diagnostic tool, with a very high false negative rate, emphasizing the need for more accurate, preferably non-invasive methods to diagnose myocarditis. Among these, antimyosin scintigraphy and Gallium-67 imaging have shown promise in detecting myocyte injury. However, these methods have low specifity and low positive predictive values [5]. Echocardiography has a limited role in the diagnosis of myocarditis, but it can help to identify with fulminant myocarditis at presentation. These patients generally have thickened myocardial walls due to edema. In some patients this increase in wall thickness may mimic hypertrophic cardiomyopathy in early course of the disease. Echocardiographic analysis of changes in myocardial texture in acute myocarditis is a new but not yet established approach. Recently, magnetic resonance imaging (MRI) and also multidetector computed tomography have shown promising results in the diagnosis and follow-up of myocarditis [4, 6–13]. The aim of the present study was to assess the MRI findings and relate them to the clinical, technical and other imaging findings in patients with a clinical suspicion of acute myocarditis.
Materials and methods
The patient group consisted of 20 patients (14 male) aging 39.7 ± 12.0 years, range 21–63, referred to MRI with presumptive, clinical diagnosis of myocarditis (Table 1). All studies were performed according to the guidelines of the hospital committee on medical ethics and clinical investigation and all subjects gave informed consent for the study.
All MRI studies were performed on a 1.5-T Intera CV MRI unit (Philips Medical Systems, Best, The Netherlands) with Powertrak 6,000 gradients (30 mT/m, 220 µs rise time), a dedicated cardiac software package and the standard five-element Synergy cardiac coil and VectorCardioGram possibilities. Imaging was started with acquisition of survey scans in three orthogonal planes to localize the heart within the chest. Afterwards, real-time interactive scanning was used to determine the intrinsic cardiac axes. Next, cine MRI was performed in the cardiac short-axis, vertical long-axis and horizontal long-axis plane, using a breath-hold balanced fast field echo sequence. Sequence parameters were TR 3.6 ms, TE 1.8 ms, flip angle 60°, slice thickness 8 mm, matrix 160×256, field of view 300 mm, FOV: 80%, number of phases: 30. Depending on the heart rate, this breath-hold acquisition, acquired at end inspiration, took 10–15 s. Cardiac short-axis slices encompassed the entire left ventricle (LV). Next, T2-short-tau inversion-recovery fast spin-echo MRI was performed in the cardiac short-axis (TR: 2 heart beats, TE: 100 ms, slice thickness: 8 mm, field-of-view: 320 mm, matrix: 256×256, rectangular FOV: 80). Finally, inversion recovery contrast enhanced MRI was performed after intravenous injection of gadopentetate dimeglumine (0.2 mmol/kg body weight), using a three-dimensional T1-weighted turbo-field echo technique in the cardiac short-axis and long-axis planes (TR 4.5 ms, TE 1.3 ms, flip angle 15°, slice thickness 10 mm, matrix 128×256, field of view 350 mm). The inversion time was adjusted for optimal suppression of normal myocardial signal (inversion time approximately 200–300 ms), and the images were obtained within 10–20 min after injection of gadolinium.
In 19/20 patients, a transthoracic echocardiography was performed. In 13 of the 20 patients, an additional coronary angiography was performed to exclude coronary artery disease. In young patients, unnecessary coronary angiographies were avoided. In only five patients, a RV EMB was performed.
MRI analysis
For assessment of global LV function, the slice-summation technique was used. The endocardial contours of all short-axis cine MR images encompassing the left ventricle were delineated at end diastole and end systole, enabling calculation of end-diastolic and end-systolic volumes, stroke volumes, and ejection fractions. Regional LV function was assessed using the 17-segment model as defined by the American Society of Echocardiography [14]. Systolic wall motion was described as normokinetic (N), slightly (H+), moderate (H++), severely hypokinetic (H+++), akinetic (A), or dyskinetic (D). Myocardial edema was defined as increased signal intensity [+2 standard deviations (SDs) above the signal intensity of normal myocardial tissue] on T2-weighted STIR fast spin-echo. A similar approach was used to define abnormal myocardial enhancement on late-enhancement MRI, and the location of enhancement wihin the myocardium was described as subendocardial, midwall, subepicardial or transmural. Presence of myocardial edema and abnormalities on late-enhancement MRI were segmentally described using the 17-segment model [14]. Also, images were analyzed for RV enhancement. The MR images were also analyzed for the presence of concomitant pericardial effusion, and enhancement of the pericardial layers on late-enhancement MRI. All results are shown as mean±SD.
Results
As shown in Table 1, acute chest pain was the most common clinical presentation (13/20 patients), often resembling ischemia-like chest pain. An obvious viral syndrome was only present in 2/20 patients. ECG findings ranged from normal (n = 4), non-specific abnormalities (n = 2), to ST-segment depression (n = 3), ST-segment elevation (n = 3), and negative T-waves (n = 10) mostly in the inferolateral LV wall (n = 13), less frequently anterior (n = 2). Coronary angiography performed in 13/20 patients, showing minimal atherosclerotic abnormalities in three patients but no signicificant coronary artery stenoses. Increased troponin I levels were found in 17/20 patients (9.7 ± 8.6 μg/l) (Table 2). Right heart EMB performed in 5/20 patients did not reveal any specific abnormalities.
Cardiac MRI findings
The average duration between symptom onset and MRI exam was 9.8 ± 7.5 days. Mean LV end-diastolic volume was 172 ± 50 ml, range 103–264. Mean ejection fraction was 57 ± 10%, range 31–76. Regional wall motion abnormalities were found on MRI in 16 of the 20 patients whereas on echocardiography only in three of the 19 patients (Table 3). In 15 of the 16 patients, there was a match between regional functional abnormalities and DE. If regional dysfunction was present, contractile abnormalities were usually mild to moderate. Akinesia or dyskinesia was never found.
Fifteen of the 20 patients displayed abnormal myocardial enhancement on DE-MRI (Tables 2, 4). The subepicardial myocardial region was the most frequent site of DE (67% of enhanced segments), less common were midwall and transmural enhancement (Figs. 1, 2). In some patients, the pattern of DE differed according the position within the left ventricle, i.e., midwall involvement in the ventricular septum and subepicardial involvement in lateral LV wall. Even though some patients showed transmural (usually patchy) enhancement, there was never isolated subendocardial DE. According to the 17-segment model, the lateral LV wall (segments 5, 6, 11, 12, 16) was most frequently involved, i.e., 32 of 55 segments with DE (Fig. 3). Involvement of the interventricular septum was found in two patients, presenting as a focal midwall enhancement (Fig. 3). Six patients presented a minor to mild amount of pericardial fluid, while in nine patients DE of the pericardial layers was found. In no patients was right ventricular DE found. In 8/16 patients, increased myocardial signal intensity was found on T2-weighted-STIR images. Though the spatial extent of abnormalities was less extensive than on delayed contrast-enhanced MRI (2.1 ± 1.1 segments versus 3.7 ± 2.1 segments), in 7/8 patients a good match in location was found with DE (Fig. 4).
Acute myocarditis in a 35-year old male presenting with acute chest pain, showing normal coronary arteries. Delayed enhancement MRI shows a strong subepicardial enhancement in the anterolateral LV wall as shown in the short-axis view (white arrows), b the spread in longitudinal direction can be well appreciated on the vertical long-axis view (white arrows)
Acute myocarditis in 39-year-old male presenting with acute chest pain. MRI shows a close matching between the abnormalities on T2-weighted STIR imaging and DE-MRI. a Short-axis T2-weighted STIR image, and b short-axis DE-MRI obtained at the same level show increased signal in the subepicardial part of the lateral LV wall. c The abnormalities are confirmed on the horizontal long-axis view
Four patients showed increased troponin I levels without DE, two patients had evidence of DE without increased troponin I levels. There was no relation between the increase in troponin I levels and presence and extent of DE. The size of the group of patients with increased troponin I levels without DE was too small to look for statistical significant differences in global and regional function with the group with increased enzyme levels and with DE.
All patients received supportive, anti-inflammatory and if necessary anti-arrhythmic and heart failure treatment. Clinical follow-up at 6 months, available for 18 patients, was uneventful for 15 patients, while three patients showed a relapse of myocarditis, respectively at 1, 3 and 5 months.
Discussion
Using a comprehensive MRI approach in patients with a clinical suspicion of acute myocarditis, a pattern of abnormalities is found that seems to be quite specific and thus might enable to differentiate it from other myocardial diseases, in particular ischemic heart disease. Most typically is the enhancement of the subepicardial part of the LV lateral wall. Not infrequently the enhancement extends beyond the outer borders of the myocardium into the adjacent pericardium (“peri-myocarditis”) often associated with a minor/mild pericardial effusion. Extension of the lateral wall enhancement anteriorly or inferiorly is common. Septal enhancement, although relatively rare, is shown as linear midwall enhancement with normal appearance of the subendocardial layers on both sides of the ventricular septum. In the current study group, no right ventricular myocardial wall enhancement was found, most likely explaining the “false” negative findings on EMB. Functionally, regional contraction abnormalities are invariably present in the areas with wall enhancement but are visually scored as mildy to moderately hypokinetic. The impact on global ventricular function is usually limited with low-normal to mildly decreased ejection fractions, and normal to mildly increased end-diastolic ventricular volumes.
These findings are in agreement with previous reports using DE-MRI to study patients with clinical suspicion with acute myocarditis [4, 9–12]. The location of enhancement within the wall and throughout the ventricles seems to be fairly specific for myocarditis. Presence of subepicardial and midwall enhancement is highly suspicous for myocarditis in a patient with clinical suspicion for myocarditis, and always enables exclusion of ischemia-related myocardial damage, since this disease starts in the subendocardium and spreads like a wavefront in the transmural direction [9]. Moreover, the lateral wall and less frequently the (basal) ventricular septum are most commonly involved. Although speculative and definitely not proven, it might be postulated that one of the reasons for subepicardial involvement in lateral LV wall might be direct extension from the adjacent pericardial sac, that at this location is very near the LV myocardium. Eventually, the pericardial sac may act as an intermediate between left lung/pleural space and myocardium. Although in the current study population there was no evidence of concomitant pulmonary infection, nine of 20 patients showed some degree of enhancement of the pericardial layers, indicating an inflammatory reaction, which may coincide or eventually precede the myocardial involvement [12, 15]. Another hypothesis may be related to lower wall stresses in the subepicardial part of the myocardium. In a recent study, Mahrholdt and co-workers found a relationship between the type of virus and pattern of myocardial damage, as well as the clinical course [12].
Presence of increased signal on T2-weighted STIR imaging is indicative of increased myocardial water content (“myocardial edema”) and is, for example, typically found in patients with a new myocardial infarction [16, 17]. Moreover, the extent is significantly larger than the area of necrosis [16]. In our study group, in less than 50% of patients abnormalities were found on T2-weighted STIR imaging, and the extent was less extensive than the DE area. Since the pathophysiological mechanism is different, this might explain the observed differences with less extensive edema in patients with acute myocarditis. Complete or partial resorption of myocardial edema between onset of symptoms and MRI exam may be another potential explanation.
In 25% of patients (five patients) no abnormalities were found neither on T2-weighted STIR nor DE imaging, although four of five patients had an abnormal troponin I level. In the study by Abdel-aty and co-workers, sensitivity, specificity and accuracy of DE for the detection of acute myocarditis were 44%, 100% and 71% respectively, in this study nearly half of the patients showed DE [10, 18]. In the study by Mahrholdt et al. [4], 88% of patients showed contrast enhancement. This can be explained in several ways. In borderline myocarditis, myocyte injury is not present and in this group it may be possible not to see delayed contrast enhancement. On the other hand, there are cytokine and humoral mediated forms of myocarditis and again these forms may not display delayed enhancement. Also, virus type can be important for the pattern of myocardial injury. The time of the MRI study after the onset of symptoms, especially for T2-weighted STIR imaging, may also be important.
Another remarkable finding is the lack of specifity of transthoracic echocardiography in assessing regional wall motion abnormality compared with cine MRI. A likely explanation is the involvement of the lateral LV wall, often more difficultly accessible with echocardiography, and the mild or moderate degree of dysfunction.
The main limitation of the current study is the lack of validation of the MRI findings. As mentioned above, the value of EMB, as the “gold standard”, is questionable unless a targeted approach is used [4], for example, using DE-MRI to guide your biopsy. The diagnosis of myocarditis was therefore based on clinical signs, coronary angiography, ECG, laboratory, enzymes and clinical follow-up.
In conclusion, cardiac MRI is an important diagnostic tool in patients with a clinical suspicion of acute myocarditis, especially in patients with unexplained chest pain, elevated cardiac enzymes and normal coronary arteries. Not only does it allow detection of the presence and extent of myocardial damage but it also allows differentiation of other entities (e.g., ischemia-related), to detect the presence of concomitant pericardial involvement and to quantify the impact on regional and global function. However, it should be emphasized that a considerable number of patients have normal MRI findings despite increased enzyme levels and ECG abnormalities. Further research is necessary to better understand and categorize these MRI-negative patients. Finally, comprehensive MRI including DE can be used to follow-up patients with acute myocarditis, and also to better understand the underlying mechanisms of the evolution towards heart failure in some patients [12, 19].
References
Braunwald E (ed) (2006) Heart disease: a textbook of cardiovascular medicine, 7th edn. Saunders, Philadelphia, pp 1699–1705
Harrison’s principles of internal medicine, 16th edn (2004) McGraw-Hill, New York, pp 1471–1472
Crawford MH (ed) (2003) Current diagnosis and treatment in cardiology, 2nd edn. McGraw-Hill, New York, pp 671–678
Mahrholdt H, Goedecke C, Wagner A et al (2004) Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation 109:1250–1258
Narula I, An Khaw B, Dec GW et al (1996) Diagnostic accuracy of antimyosin scintigraphy in suspected myocarditis. J Nucl Cardiol 3:3781–3787
Matsouka H, Hamad M, Honda T et al (1994) Evaluation of acute myocarditis and pericarditis by Gd-DTPA enhanced magnetic resonance imaging 15:283–288
Friedrich MG, Strohm O, Schulz-Menger J, Marciniak H, Luft FC, Dietz R (1998) Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation 97:1802–1809
Laissy JP, Messin B, Varenne O et al (2002) MRI of acute myocarditis: a comprehensive approach based on various imaging sequences. Chest 122:1638–1648
Laissy JP, Hyafil F, Feldman LJ et al (2005) Differentiating acute myocardial infarction from myocarditis: diagnostic value of early-and delayed perfusion cardiac MR imaging. Radiology 237:75–82
Abdel-Aty H, Boye P, Zagrosek A et al (2005) Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol 7:1815–1822
Dambrin G, Laissy JP, Serfaty JM, Caussin C, Lancelin B, Paul JF (2007) Diagnostic value of ECG-gated multidetector computed tomography in the early phase of suspected acute myocarditis. A preliminary comparative study with cardiac MRI. Eur Radiol 17:331–338
Mahrholdt H, Wagner A, Deluigi CC et al (2006) Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 114:1581–1590
Bogaert J, Dymarkowski S (2005) Delayed contrast-enhanced MRI: use in myocardial viability assessment and other cardiac pathology. Eur Radiol 15(Suppl 2):B52–B58
Cerqueira MD, Weissman NJ, Dilsizian V et al (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. Circulation 105:539–542
Taylor AM, Dymarkowski S, Verbeken E, Bogaert J (2006) Detection of acute and chronic pericardial inflammation with inversion-recovery contrast-enhanced MRI: initial results. Eur Radiol 16:569–574
Aletras AH, Tilak GS, Natanzon A et al (2006) Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging. Histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation 113:1865–1870
Stork A, Lund GK, Muellerleile K et al (2006) Characterization of the peri-infarction zone using T2-weighted MRI and delayed-enhancement MRI in patients with acute myocardial infarction. Eur Rad 16:2350–2357
Liu PP, Yan AT (2005) Cardiovascular magnetic resonance for the diagnosis of acute myocarditis: prospects for detecting myocardial inflammation. J Am Coll Cardiol 45:1823–1825
De Cobelli F, Pieroni M, Esposito A et al (2006) Delayed gadolinium-enhanced cardiac magnetic resonance in patients with chronic myocarditis presenting with heart failure or recurrent arrhythmias. J Am Coll Cardiol 18:1649–1654
Acknowledgements
The authors would like to thank Jan Dhooghe, PhD, for helpful comments in preparing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 2.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by-nc/2.0/.
About this article
Cite this article
Yelgec, N.S., Dymarkowski, S., Ganame, J. et al. Value of MRI in patients with a clinical suspicion of acute myocarditis. Eur Radiol 17, 2211–2217 (2007). https://doi.org/10.1007/s00330-007-0612-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-007-0612-3