Abstract
Background
Patient-reported outcomes (PROs) are of growing importance in research and clinical care and may be used as primary outcomes or as compliments to traditional surgical outcomes. In assessing the impact of surgical and traumatic scars, PROs are often the most meaningful. To assess outcomes from the patient perspective, rigorously developed and validated PRO instruments are essential.
Methods
The authors conducted a systematic literature review to identify PRO instruments developed and/or validated for patients with surgical and/or non-burn traumatic scars. Identified instruments were assessed for content, development process, and validation under recommended guidelines for PRO instrument development.
Results
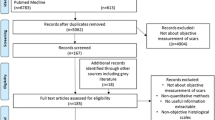
The systematic review identified 6534 articles. After review, we identified four PRO instruments meeting inclusion criteria: patient and observer scar assessment scale (POSAS), bock quality of life questionnaire for patients with keloid and hypertrophic scarring (Bock), patient scar assessment questionnaire (PSAQ), and patient-reported impact of scars measure (PRISM). Common concepts measured were symptoms and psychosocial well-being. Only PSAQ had a dedicated appearance domain. Qualitative data were used to inform content for the PSAQ and PRISM, and a modern psychometric approach (Rasch Measurement Theory) was used to develop PRISM and to test POSAS. Overall, PRISM demonstrated the most rigorous design and validation process, however, was limited by the lack of a dedicated appearance domain.
Conclusions
PRO instruments to evaluate outcomes in scars exist but vary in terms of concepts measured and psychometric soundness. This review discusses the strengths and weaknesses of existing instruments, highlighting the need for future scar-focused PRO instrument development.
Level of Evidence IV
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to Table of Contents or the online Instructions to Authors www.springer.com/00266.

Similar content being viewed by others
References
Weiser TG, Regenbogen SE, Thompson KD, Haynes AB, Lipsitz SR, Berry WR, Gawande AA (2008) An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 372:139–144
Brown BC, Mckenna SP, Siddhi K, Mcgrouther DA, Bayat A (2008) The hidden cost of skin scars: quality of life after skin scarring. J Plast Reconstr Aesthet Surg 61:1049–1058
Kinahan KE, Sharp LK, Seidel K, Leisenring W, Didwania A, Lacouture ME, Stovall M, Haryani A, Robison LL, Krull KR (2012) Scarring, disfigurement, and quality of life in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol 30:2466–2474
Sullivan T, Smith J, Kermode J, Mciver E, Courtemanche DJ (1990) Rating the burn scar. J Burn Care Rehabil 11:256–260
Beausang E, Floyd H, Dunn KW, Orton CI, Ferguson MW (1998) A new quantitative scale for clinical scar assessment. Plast Reconstr Surg 102:1954–1961
Fda (2014) Clinical Outcome Assessment Qualification Program. Journal 2015
US Food and Drug Administration (2009) Guidance for industry patient-reported outcome measures: use in medical product development to support labeling claims. http://www.fda.gov/downloads/Drugs/…/Guidances/Ucm193282.Pdf. Accessed 11 Aug 2014
Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, Bouter LM, De Vet HC (2010) The Cosmin checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res 19:539–549
Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, Bouter LM, De Vet, HC (2012) Cosmin checklist manual. http://www.Cosmin.Nl/Images/Upload/Files/Cosminchecklistmanualv9.Pdf. Jan 2012
Aaronson N, Alonso J, Burnam A, Lohr KN, Patrick DL, Perrin E, Stein RE (2002) Assessing health status and quality-of-life instruments: attributes and review criteria. Qual Life Res 11:193–205
Klassen AF, Stotland MA, Skarsgard ED, Pusic AL (2008) Clinical research in pediatric plastic surgery and systematic review of quality-of-life questionnaires. Clin Plast Surg 35:251–267
Singh SJ, Sodergren SC, Hyland ME, Williams J, Morgan MD (2001) A comparison of three disease-specific and two generic health-status measures to evaluate the outcome of pulmonary rehabilitation in COPD. Respir Med 95:71–77
Draaijers LJ, Tempelman FR, Botman YA, Tuinebreijer WE, Middelkoop E, Kreis RW, Van Zuijlen PP (2004) The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg 113:1960–1965 discussion 1966–1967
Bock O, Schmid-Ott G, Malewski P, Mrowietz U (2006) Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res 297:433–438
Durani P, Mcgrouther DA, Ferguson MW (2009) The patient scar assessment questionnaire: a reliable and valid patient-reported outcomes measure for linear scars. Plast Reconstr Surg 123:1481–1489
Brown BC, Mckenna SP, Solomon M, Wilburn J, Mcgrouther DA, Bayat A (2010) The patient-reported impact of scars measure: development and validation. Plast Reconstr Surg 125:1439–1449
Van De Kar AL, Corion LU, Smeulders MJ, Draaijers LJ, Van Der Horst CM, Van Zuijlen PP (2005) Reliable and feasible evaluation of linear scars by the patient and observer scar assessment scale. Plast Reconstr Surg 116:514–522
Nicholas RS, Falvey H, Lemonas P, Damodaran G, Ghanem AM, Selim F, Navsaria H, Myers S (2012) Patient-related keloid scar assessment and outcome measures. Plast Reconstr Surg 129:648–656
Van Der Wal MBA, Tuinebreijer WE, Bloemen MCT, Verhaegen P, Middelkoop E, Van Zuijlen PPM (2012) Rasch analysis of the Patient and Observer Scar Assessment Scale (POSAS) in burn scars. Qual Life Res 21:13–23
Van Der Wal MB, Tuinebreijer WE, Lundgren-Nilsson A, Middelkoop E, Van Zuijlen PP (2014) Differential item functioning in the observer scale of the POSAS for different scar types. Qual Life Res 23:2037–2045
Durani P, Mcgrouther DA, Ferguson MW (2009) Current scales for assessing human scarring: a review. J Plast Reconstr Aesthet Surg 62:713–720
Tyack Z, Simons M, Spinks A, Wasiak J (2012) A systematic review of the quality of burn scar rating scales for clinical and research use. Burns 38:6–18
Schmid-Ott G, Burchard R, Niederauer HH, Lamprecht F, Kunsebeck HW (2003) Stigmatization and quality of life of patients with psoriasis and atopic dermatitis. Hautarzt 54:852–857
Ginsburg IH, Link BG (1989) Feelings of stigmatization in patients with psoriasis. J Am Acad Dermatol 20:53–63
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Mundy, L.R., Miller, H.C., Klassen, A.F. et al. Patient-Reported Outcome Instruments for Surgical and Traumatic Scars: A Systematic Review of their Development, Content, and Psychometric Validation. Aesth Plast Surg 40, 792–800 (2016). https://doi.org/10.1007/s00266-016-0642-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-016-0642-9