Abstract
Objectives
Scientific advances in the last decade have highlighted the use of immunotherapy, especially immune checkpoint inhibitors, to be an effective strategy in cancer therapy. However, these immunotherapeutic agents are expensive, and their use must take into account economic criteria. Thus, the objective of the present study was to systematically identify and review published EE related to the use of ipilimumab, nivolumab or pembrolizumab in melanoma, lung cancer, head and neck cancer or renal cell carcinoma, and to assess their quality.
Methods
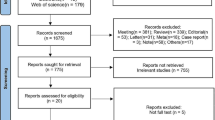
The systematic literature research was conducted on Medline via PubMed and the Cochrane Central Register of Controlled Trials to identify economic evaluations published before July 2018. The quality of each selected economic evaluation was assessed by two independent reviewers using the Drummond checklist.
Results
Our systematic review was based on 32 economic evaluations using different methodological approaches, different perspectives and different time horizons. Three-quarters of the economic evaluations are full (n = 24) with a Drummond score ≥ 7, synonymous of “high quality”. Among them, 66% reported a strategy that was cost-effective. The most assessed immunotherapeutic agent was nivolumab. In patients with renal cell carcinoma or head and neck cancer, it was less likely to be cost-effective than in patients with melanoma or lung cancer.
Conclusions
Whether or not these findings will be confirmed remains to be seen when market approval to cover more indications is extended and new effective immunotherapeutic agents become available.


Similar content being viewed by others
Abbreviations
- CEA:
-
Cost-effectiveness analyses
- CUA:
-
Cost-utility analyses
- EE:
-
Economic evaluation
- HRQOL:
-
Health-related quality of life
- HSUV:
-
Health-state utility values
- HTA:
-
Health technology assessment
- LY:
-
Life years
- MeSH:
-
Medical subject headings
- QALY:
-
Quality-adjusted life year
- UK:
-
United Kingdom
References
Finn OJ (2012) Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol 23(Suppl 8):viii6–viii9. https://doi.org/10.1093/annonc/mds256
Brown JA, Dorfman DM, Ma F-R et al (2003) Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol 170:1257–1266. https://doi.org/10.4049/jimmunol.170.3.1257
Leach DR, Krummel MF, Allison JP (1996) Enhancement of antitumor immunity by CTLA-4 blockade. Science 271:1734–1736. https://doi.org/10.1126/science.271.5256.1734
Freeman GJ, Long AJ, Iwai Y et al (2000) Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192:1027–1034. https://doi.org/10.1084/jem.192.7.1027
Hodi FS, O’Day SJ, McDermott DF et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723. https://doi.org/10.1056/NEJMoa1003466
Larkin J, Chiarion-Sileni V, Gonzalez R et al (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373:23–34. https://doi.org/10.1056/NEJMoa1504030
Robert C, Schachter J, Long GV et al (2015) Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372:2521–2532. https://doi.org/10.1056/NEJMoa1503093
Weber JS, D’Angelo SP, Minor D et al (2015) Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 16:375–384. https://doi.org/10.1016/S1470-2045(15)70076-8
Brahmer J, Reckamp KL, Baas P et al (2015) Nivolumab versus Docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123–135. https://doi.org/10.1056/NEJMoa1504627
Borghaei H, Paz-Ares L, Horn L et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639. https://doi.org/10.1056/NEJMoa1507643
Reck M (2018) Pembrolizumab as first-line therapy for metastatic non-small-cell lung cancer. Immunotherapy 10:93–105. https://doi.org/10.2217/imt-2017-0121
Herbst RS, Baas P, Kim D-W et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387:1540–1550. https://doi.org/10.1016/S0140-6736(15)01281-7
Motzer RJ, Escudier B, McDermott DF et al (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1803–1813. https://doi.org/10.1056/NEJMoa1510665
Ferris RL, Blumenschein G, Fayette J et al (2016) Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 375:1856–1867. https://doi.org/10.1056/NEJMoa1602252
Vaughn DJ, Bellmunt J, Fradet Y et al (2018) Health-related quality-of-life analysis from KEYNOTE-045: a phase iii study of pembrolizumab versus chemotherapy for previously treated advanced urothelial cancer. J Clin Oncol 36:1579–1587. https://doi.org/10.1200/JCO.2017.76.9562
European Medicines Agency. https://www.ema.europa.eu/ema/. Accessed 16 Sep 2019
French health insurance. https://ameli.fr. Accessed 16 Sep 2019
Schnipper LE, Davidson NE, Wollins DS et al (2015) American Society of Clinical Oncology Statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol 33:2563–2577. https://doi.org/10.1200/JCO.2015.61.6706
Cochrane Handbook for Systematic Reviews of Interventions. https://handbook-5-1.cochrane.org/. Accessed 16 Sep 2019
Drummond MF, Sculpher MJ, Claxton K et al (2015) Methods for the economic evaluation of health care programmes. Oxford University Press, Oxford
Whitehead SJ, Ali S (2010) Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull 96:5–21. https://doi.org/10.1093/bmb/ldq033
Husereau D, Drummond M, Petrou S et al (2013) Consolidated Health Economic Evaluation Reporting Standards (CHEERS)–explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 16:231–250. https://doi.org/10.1016/j.jval.2013.02.002
The Fed—Foreign Exchange Rates—G.5A Annual. https://www.federalreserve.gov/releases/g5a/current/default.htm. Accessed 6 Mar 2019
Zargar M, McFarlane T, Chan KKW, Wong WWL (2018) Cost-effectiveness of nivolumab in recurrent metastatic head and neck squamous cell carcinoma. Oncologist 23:225–233. https://doi.org/10.1634/theoncologist.2017-0277
Tringale KR, Carroll KT, Zakeri K et al (2018) Cost-effectiveness analysis of nivolumab for treatment of platinum-resistant recurrent or metastatic squamous cell carcinoma of the head and neck. J Natl Cancer Inst 110:479–485. https://doi.org/10.1093/jnci/djx226
Ward MC, Shah C, Adelstein DJ et al (2017) Cost-effectiveness of nivolumab for recurrent or metastatic head and neck cancer☆. Oral Oncol 74:49–55. https://doi.org/10.1016/j.oraloncology.2017.09.017
Goeree R, Villeneuve J, Goeree J et al (2016) Economic evaluation of nivolumab for the treatment of second-line advanced squamous NSCLC in Canada: a comparison of modeling approaches to estimate and extrapolate survival outcomes. J Med Econ 19:630–644. https://doi.org/10.3111/13696998.2016.1151432
Matter-Walstra K, Schwenkglenks M, Aebi S et al (2016) A cost-effectiveness analysis of nivolumab versus docetaxel for advanced nonsquamous NSCLC including PD-L1 testing. J Thorac Oncol 11:1846–1855. https://doi.org/10.1016/j.jtho.2016.05.032
Huang M, Lou Y, Pellissier J et al (2017) Cost-effectiveness of pembrolizumab versus docetaxel for the treatment of previously treated PD-L1 positive advanced NSCLC patients in the United States. J Med Econ 20:140–150. https://doi.org/10.1080/13696998.2016.1230123
Huang M, Lou Y, Pellissier J et al (2017) Cost effectiveness of pembrolizumab vs. standard-of-care chemotherapy as first-line treatment for metastatic NSCLC that expresses high levels of PD-L1 in the United States. PharmacoEconomics 35:831–844. https://doi.org/10.1007/s40273-017-0527-z
Aguiar PN, Perry LA, Penny-Dimri J et al (2017) The effect of PD-L1 testing on the cost-effectiveness and economic impact of immune checkpoint inhibitors for the second-line treatment of NSCLC. Ann Oncol 28:2256–2263. https://doi.org/10.1093/annonc/mdx305
Barzey V, Atkins MB, Garrison LP et al (2013) Ipilimumab in 2nd line treatment of patients with advanced melanoma: a cost-effectiveness analysis. J Med Econ 16:202–212. https://doi.org/10.3111/13696998.2012.739226
Jarkowski A, Nestico JS, Vona KL, Khushalani NI (2014) Dose rounding of ipilimumab in adult metastatic melanoma patients results in significant cost savings. J Oncol Pharm Pract 20:47–50. https://doi.org/10.1177/1078155213476723
Curl P, Vujic I, van’t Veer LJ et al (2014) Cost-effectiveness of treatment strategies for BRAF-mutated metastatic melanoma. PLoS ONE 9:e107255. https://doi.org/10.1371/journal.pone.0107255
Yousaf N, Davidson M, Goode E et al (2015) The cost of ipilimumab toxicity: a single-centre analysis. Melanoma Res 25:259–264. https://doi.org/10.1097/CMR.0000000000000158
Toy EL, Vekeman F, Lewis MC et al (2015) Costs, resource utilization, and treatment patterns for patients with metastatic melanoma in a commercially insured setting. Curr Med Res Opin 31:1561–1572. https://doi.org/10.1185/03007995.2015.1062356
Chang C-L, Schabert VF, Munakata J et al (2015) Comparative healthcare costs in patients with metastatic melanoma in the USA. Melanoma Res 25:312–320. https://doi.org/10.1097/CMR.0000000000000159
Guglieri-López B, Pérez-Pitarch A, Porta Oltra B et al (2016) Effectiveness, toxicity, and economic evaluation of ipilimumab for the treatment of patients with metastatic melanoma in the Spanish outpatient setting. Anticancer Drugs 27:679–684. https://doi.org/10.1097/CAD.0000000000000368
Jensen I, Zacherle E, Blanchette C et al (2016) Evaluating cost benefits of combination therapies for advanced melanoma. Drugs Context 5:1–14. https://doi.org/10.7573/dic.212297
Bohensky MA, Pasupathi K, Gorelik A et al (2016) A cost-effectiveness analysis of nivolumab compared with ipilimumab for the treatment of BRAF wild-type advanced melanoma in Australia. Value Health 19:1009–1015. https://doi.org/10.1016/j.jval.2016.05.013
Russi A, Chiarion-Sileni V, Damuzzo V et al (2017) Case study on an ipilimumab cost-containment strategy in an Italian hospital. Int J Technol Assess Health Care 33:199–205. https://doi.org/10.1017/S0266462317000332
Oh A, Tran DM, McDowell LC et al (2017) Cost-effectiveness of nivolumab-ipilimumab combination therapy compared with monotherapy for first-line treatment of metastatic melanoma in the United States. J Manag Care Spec Pharm 23:653–664. https://doi.org/10.18553/jmcp.2017.23.6.653
Miguel LS, Lopes FV, Pinheiro B et al (2017) Cost effectiveness of pembrolizumab for advanced melanoma treatment in Portugal. Value Health 20:1065–1073. https://doi.org/10.1016/j.jval.2017.05.009
Wang J, Chmielowski B, Pellissier J et al (2017) Cost-effectiveness of pembrolizumab versus ipilimumab in ipilimumab-naïve patients with advanced melanoma in the United States. J Manag Care Spec Pharm 23:184–194. https://doi.org/10.18553/jmcp.2017.23.2.184
Kohn CG, Zeichner SB, Chen Q et al (2017) Cost-effectiveness of immune checkpoint inhibition in BRAF wild-type advanced melanoma. J Clin Oncol 35:1194–1202. https://doi.org/10.1200/JCO.2016.69.6336
Sarfaty M, Leshno M, Gordon N et al (2018) Cost effectiveness of nivolumab in advanced renal cell carcinoma. Eur Urol 73:628–634. https://doi.org/10.1016/j.eururo.2017.07.041
Wan XM, Peng LB, Ma JA, Li YJ (2017) Economic evaluation of nivolumab as a second-line treatment for advanced renal cell carcinoma from US and Chinese perspectives: cost of nivolumab for renal cell carcinoma. Cancer 123:2634–2641. https://doi.org/10.1002/cncr.30666
Meng Y, Hertel N, Ellis J et al (2018) The cost-effectiveness of nivolumab monotherapy for the treatment of advanced melanoma patients in England. Eur J Health Econ 19:1163–1172. https://doi.org/10.1007/s10198-018-0964-4
Shafrin J, Skornicki M, Brauer M et al (2018) An exploratory case study of the impact of expanding cost-effectiveness analysis for second-line nivolumab for patients with squamous non-small cell lung cancer in Canada: does it make a difference? Health Policy 122:607–613. https://doi.org/10.1016/j.healthpol.2018.04.008
Meng J, Lister J, Vataire A-L et al (2018) Cost-effectiveness comparison of cabozantinib with everolimus, axitinib, and nivolumab in the treatment of advanced renal cell carcinoma following the failure of prior therapy in England. ClinicoEcon Outcomes Res 10:243–250. https://doi.org/10.2147/CEOR.S159833
Raphael J, Sun Z, Bjarnason GA et al (2018) Nivolumab in the treatment of metastatic renal cell carcinoma: a cost-utility analysis. Am J Clin Oncol. https://doi.org/10.1097/COC.0000000000000451
McCrea C, Johal S, Yang S, Doan J (2018) Cost-effectiveness of nivolumab in patients with advanced renal cell carcinoma treated in the United States. Exp Hematol Oncol. https://doi.org/10.1186/s40164-018-0095-8
Lee D, Amadi A, Sabater J et al (2018) Can we accurately predict cost effectiveness without access to overall survival data? The case study of nivolumab in combination with ipilimumab for the treatment of patients with advanced melanoma in England. Pharmacoecon Open 3:43–54. https://doi.org/10.1007/s41669-018-0080-5
Swallow E, Messali A, Ghate S et al (2018) The additional costs per month of progression-free survival and overall survival: an economic model comparing everolimus with cabozantinib, nivolumab, and axitinib for second-line treatment of metastatic renal cell carcinoma. J Manag Care Spec Pharm 24:335–343. https://doi.org/10.1855/jmcp.2018.24.4.335
Sarfaty M, Hall PS, Chan KKW et al (2018) Cost-effectiveness of pembrolizumab in second-line advanced bladder cancer. Eur Urol 74:57–62. https://doi.org/10.1016/j.eururo.2018.03.006
Shea BJ, Grimshaw JM, Wells GA et al (2007) Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 7:10. https://doi.org/10.1186/1471-2288-7-10
Verma V, Sprave T, Haque W et al (2018) A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. J Immunother Cancer 6:128. https://doi.org/10.1186/s40425-018-0442-7
Tarhini A, Benedict A, McDermott D et al (2018) Sequential treatment approaches in the management of BRAF wild-type advanced melanoma: a cost-effectiveness analysis. Immunotherapy 10:1241–1252. https://doi.org/10.2217/imt-2018-0085
Funding
No sources of funding were used to assist in the preparation of this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: VN, PF; Methodology: VN, PF, SL; Writing: CC, FP, MK, VN; Literature search: CC, PF; Critically revised work: FA, VW, TM, ATV, CB, MK; Supervision: VN.
Corresponding author
Ethics declarations
Conflict of interest
C. Couchoud, P. Fagnoni, C. Gérard, M. Kroemer and S. Limat have declared no conflicts of interest. F. Aubin is a consultant or investigator for BMS, MSD, Roche, GSK, Novartis, Amgen, and Pierre Fabre and has received support for congresses from BMS, MSD, Novartis and Roche. V. Westeel has received honoraria from Astra Zeneca, BMS, MSD and Roche. T. Maurina has received honoraria from Ipsen, Roche, Janssen and Sanofi. A. Thiery-Vuillemin has received honoraria from Pfizer, Astra Zeneca, Roche, BMS and MSD. C. Borg is an expert for Sanofi, Byer, Servier and MSD. V. Nerich has received honoraria from BMS, Pfizer, Roche, and Sanofi.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Couchoud, C., Fagnoni, P., Aubin, F. et al. Economic evaluations of cancer immunotherapy: a systematic review and quality evaluation. Cancer Immunol Immunother 69, 1947–1958 (2020). https://doi.org/10.1007/s00262-020-02646-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02646-0