Abstract
Purpose
The purpose of this retrospective, multicenter study was to assess efficacy of PSMA-PET/CT-guided salvage radiotherapy (sRT) in patients with recurrent or persistent PSA after primary surgery and PSA levels < 0.2 ng/ml.
Methods
The study included patients from a pooled cohort (n = 1223) of 11 centers from 6 countries. Patients with PSA levels > 0.2 ng/ml prior to sRT or without sRT to the prostatic fossa were excluded. The primary study endpoint was biochemical recurrence-free survival (BRFS) and BR was defined as PSA nadir after sRT + 0.2 ng/ml. Cox regression analysis was performed to assess the impact of clinical parameters on BRFS. Recurrence patterns after sRT were analyzed.
Results
The final cohort consisted of 273 patients; 78/273 (28.6%) and 48/273 (17.6%) patients had local or nodal recurrence on PET/CT. The most frequently applied sRT dose to the prostatic fossa was 66–70 Gy (n = 143/273, 52.4%). SRT to pelvic lymphatics was delivered in 87/273 (31.9%) patients and androgen deprivation therapy was given to 36/273 (13.2%) patients. After a median follow-up time of 31.1 months (IQR: 20–44), 60/273 (22%) patients had biochemical recurrence. The 2- and 3-year BRFS was 90.1% and 79.2%, respectively. The presence of seminal vesicle invasion in surgery (p = 0.019) and local recurrences in PET/CT (p = 0.039) had a significant impact on BR in multivariate analysis. In 16 patients, information on recurrence patterns on PSMA-PET/CT after sRT was available and one had recurrent disease inside the RT field.
Conclusion
This multicenter analysis suggests that implementation of PSMA-PET/CT imaging for sRT guidance might be of benefit for patients with very low PSA levels after surgery due to promising BRFS rates and a low number of relapses within the sRT field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biochemical recurrence (BR) post-radical prostatectomy (RP) occurs in approximately 30% of prostate cancer patients [1]. Before the incorporation of prostate-specific membrane antigen positron emission tomography/computed tomography (PSMA-PET/CT) imaging for salvage radiotherapy (sRT) guidance, several studies reported favorable metastasis-free survival (MFS) rates for the conduction of sRT at very low prostate-specific antigen (PSA) levels (< 0.2 ng/ml) [2, 3]. Furthermore, the prospective randomized controlled RADICALS trial compared adjuvant radiotherapy with very early sRT [4]. The authors included patients for very early sRT after three consecutive PSA rises or at PSA > 0.1 ng/ml and two consecutive rises. Consequently, current national comprehensive cancer network guidelines (NCCNv2023.1) recommend the conduction of very early sRT at PSA levels > 0.1 ng/ml or at two consecutive rises.
Recently, PSMA-PET/CT has been incorporated as a sensitive tool to detect recurrent lesions after prostatectomy. The detection rate of PSMA-PET/CT positive lesions increases at higher PSA serum levels. For example, in a prospective analysis including 635 patients, the detection rates were 97%, 86%, 84%, 57%, and 38% for PSA levels of ≥ 5, 2 to < 5, 1 to < 2, 0.5 to < 1, and 0.2 to < 0.5 ng/ml, respectively [5]. PSMA-PET/CT is not routinely used for staging patients with BR at low PSA serum levels (< 0.2 ng/ml). However, two recent prospective studies including patients with PSA < 0.2 ng/ml reported a detection rate of 51.2% [6] and 44.8% [7], respectively.
Regarding the discrepancy between low PSMA-PET/CT detection levels and favorable sRT results, there is no consensus, at present, about the role of PSMA-PET/CT imaging in patients with BR and PSA levels < 0.2 ng/ml after RP. Thus, the main aim was to assess the outcome after PSMA-PET/CT-based sRT in terms of biochemical recurrence-free survival (BRFS). In addition, univariate and multivariate Cox regression analyses have been used to assess the impact of several risk factors on PSA relapse. Third, recurrence patterns after sRT have been described.
Materials and methods
Patients
Patient data for this retrospective study was extracted and pooled for analysis from 11 medical centers from 6 countries and local ethics committees from all institutions gave their approval. The inclusion criteria for the pooled database (n = 1223) consisted of patients who underwent open or laparoscopic RP and subsequently received PSMA-PET/CT-based sRT for a PSA persistence or recurrence (PSA after prostatectomy ≥ 0.1 ng/ml for both). To create the subgroup for this analysis, patients who had PSA > 0.2 ng/ml (n = 942), who did not receive RT to all PET positive lesions (n = 2) and RT was not performed to the prostatic fossa (n = 4), were excluded. Finally, 273 patients met the inclusion criteria.
PSMA-PET/CT/CT scans prior to sRT
PET/CT prior to sRT were performed with 68 Ga-PSMA-11 (n = 224), 68 Ga-PSMA-I&T (n = 5), 18F-PSMA-1007 (n = 32), 18F-siPSMA-14 (n = 9), or 18F-PSMA-rhPSMA-7/-7.3 (n = 3) according to PSMA-PET/CT imaging protocols [8]. In all centers, two readers were assigned for the interpretation of the imaging findings. Please see Supplementary Table 1 for a detailed description of the imaging protocols of all centers.
Treatment
The treating radiation oncologists were responsible for the clinical management decisions according to the basis of standards of care at the time of treatment at each respective institution based on the decisions of multidisciplinary tumor boards [8]. Based on PSMA-PET/CT findings and individual patients’ risk factors, treatment has been individualized in terms of sRT region (prostatic fossa ± pelvic lymph nodes), sRT dose as well as inclusion and duration of androgen deprivation therapy (ADT). All patients received intensity-modulated and image-guided sRT to the prostatic fossa and boost irradiation of potentially existent local recurrences dependent on institutional clinical practice. Please see Supplementary Table 2 for a detailed description of the sRT protocols of all centers.
Follow-up
According to each medical center’s clinical practice, patients underwent follow-up assessments with serum PSA testing at regular intervals and re-staging with medical imaging in terms of biochemical relapse [8]. Please see Supplementary Table 3 for a detailed description of the follow-up protocols of all centers.
Statistical analysis
The primary study endpoint was biochemical recurrence-free survival, which was defined as PSA nadir after sRT + 0.2 ng/ml or death of any cause. Following BR after sRT, re-staging was performed with imaging (PSMA-PET/CT or CT scans with bone scintigraphy). For the graphical representation, the respective parameters were analyzed by Kaplan–Meier survival curve compared by log-rank test. Uni- and multivariate Cox regression analyses were performed to assess the impact of the different variables on BR. The multivariate Cox regression analysis included significant variables in univariate analysis. In addition, a multivariate logistic regression (LR) analysis was performed to assess the impact of clinical parameters as well as the PET tracers on the detection rate of PSMA-PET/CT imaging after prostatectomy. A two-sided p value < 0.05 was considered as statistically significant. The statistical analysis was conducted using SPSS software version: 28.0.1.1. (IBM, USA).
Results
Baseline patient and treatment characteristics
The baseline patient and treatment characteristics of the total cohort are listed in Tables 1 and 2. For the total cohort, 97/273 (35.5%) patients had extracapsular disease and 54/273 (19.8%) patients had seminal vesicle invasion in surgery. The median PSA value before sRT was 0.15 (IQR: 0.1–0.18) ng/ml. ADT was given to 36/273 (13.2%) patients. The most frequently applied equivalent dose in 2 Gy per fraction (EQD2, α/β = 1.6 Gy [9]) to the prostatic fossa was 66–70 Gy (n = 143/273, 52.4%). SRT to lymph nodes and to elective pelvic lymphatics was delivered in 20/273 (7.3%) and 87/273 (31.9%) patients, respectively.
Detection rate
For the entire cohort, 118/273 (43.2%) patients had recurrent disease detected with PSMA PET scan before sRT. More specifically, 78/273 (28.6%) patients had local recurrence and 48/273 (17.6%) nodal recurrence, respectively. However, in multivariate LR, none of the clinical parameters had statistically significant impact on the detection rate (Supplementary Table 4), whereas patients with 68 Ga-PSMA-11 had a significant lower detection rate compared to patients with 18F-PSMA-1007 (OR = 2.204, 95% CI: 1.444–3.363, p < 0.001).
Outcome
After a median follow-up time of 31.1 months (IQR: 20–44), 60/273 (22%) patients had biochemical recurrence. One patient deceased due to progressive PCa 59 months after BR. At 2 and 3 years, the estimated rates of BRFS were 90.1% and 79.2%, respectively. The detailed results of the Cox regression analyses are listed in Table 3. The presence of seminal vesicle invasion in surgery (HR = 1.945, 95% CI: 1.114–3.393, p = 0.019) and local recurrences in PET/CT (HR = 0.488, 95% CI: 0.246–0.945, p = 0.039) had a significant impact on BRFS in univariate and multivariate analysis. Please see Fig. 1 for the Kaplan–Meier representation of both risk factors. Two representative patient cases are presented in Fig. 2. There was no significant difference in BRFS (HR = 0.554, 95% CI: 0.204–1.504, p = 0.246) between patients which were staged with the 18F-PSMA-1007 or 68 Ga-PSMA-11 tracer in Cox regression analysis.
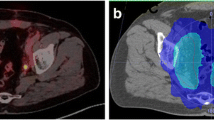
Case reports. This figure shows the treatment plans of two exemplary patients on axial and sagittal planning CT slides. The planning target volume is shown in pink, the rectum in brown, and the bladder in yellow. Radiotherapy isodoses are shown in colorwash according to the legend. Patient A: the patient presented with the following clinical characteristics after definitive prostatecomy: pT2b pN0 (0/26), L0 V0 Pn1 R1, ISUP 3, iPSA 8.5 ng/ml. Before sRT, mpMRI and PSMA-PET did not show any suspicious lesions within or outside the prostate bed. PSA prior to sRT was 0.18 ng/ml. sRT was delivedered to the prostate bed in 1.8 Gy per fraction up to 66.6 Gy. At 44 months of follow-up, no recurrent disease could be observed. Patient B: the patient presented with the following clinical characteristics after definitive prostatectomy: pT3b pN1 (3/25), L1 V0 Pn1 R1, ISUP 3, IPSA 13.8 ng/ml. Before sRT, mpMRI and PSMA-PET did not show any suscipious lesions within or outside the prostate bed. sRT was deleivered to the prostate bed in 1.8 Gy per fraction up to 66.6 Gy. Elective pelvis were treated with 1.8 Gy per fraction up to 45 Gy. Regions with positive lymph nodes were escalated with boost of 1.8 Gy per fraction up to 54 Gy. Androgen deprivation therapy was given for 6 months. After 31 months, the patient experienced a biochemical recurrence. PSMA-PET/CT imaging revealed a bone metastasis in the breast vertebrae 7
Recurrence patterns
In 48/60 (80%) patients with PSA relapse after sRT, a second PSMA-PET/CT scan was conducted and information on recurrence patterns was available in 16 patients. Local relapse was detected in three patients and four patients had positive pelvic lymph nodes. Distant metastases were observed in 10 patients, and of these, 3 patients had lymph nodes outside the pelvis and 7 had bone metastases. Recurrent disease after sRT was localized outside the RT field in 15 of the 16 patients (93.8%).
Discussion
PSMA-PET/CT imaging is increasingly used to guide sRT after prostatectomy mostly at PSA levels > 0.2 ng/ml. However, several studies suggested a significant improvement in BRFS or MFS when sRT is performed at low (< 0.2 ng/ml) PSA levels [2]. On the contrary, the detection rate of PSMA-PET/CT imaging was reported to be low in this patient population [6, 7]. In our study, we retrospectively included 273 patients with PSA ≤ 0.20 ng/ml and observed a detection rate of 43.2%. Gupta et al. [10] examined a subgroup of patients with prior prostatectomy and PSA levels ≤ 0.20 ng/ml at biochemical relapse. The authors demonstrated a detection rate of 46%. According to a meta-analysis [11], 68 Ga-PSMA-11 PET/CT detection rates for patients with PSA levels of < 0.2 ng/ml after prostatectomy ranged from 11.3 to 58.3%. Thus, the detection rate in our study is comparable to other studies, and it can be assumed that approximately one-third of the patients in this patient population might receive a change in sRT treatment concept based on PSMA-PET/CT findings, like a RT boost to the prostatic fossa, inclusion of the pelvic lymphatics, or administration of ADT. In our study, ADT was given to 13% of patients and elective pelvic lymphatics were treated in 32% of patients. Whether a treatment individualization based on PET/CT findings impacts the oncologic outcome in patients with PSA ≤ 0.20 ng/ml was not examined in current literature.
Consequently, the main aim of this study was to assess the outcome after PSMA-PET/CT-based sRT in terms of BRFS reported as 90.1% and 79.2% two and three years after sRT. Several retrospective studies reported the BRFS after early sRT in the pre-PSMA-PET/CT era. Abhugarib et al. [3] included 657 patients and reported a 2-year BRFS of 85%. Similar results were observed by Tendulkar et al. [2] with an estimated BRFS of 82% two years after sRT. A direct comparison of the BRFS rates between our study and the two others is hampered by different patient cohorts, follow-up protocols, and definition of BR. However, our results suggest that the induction of PSMA-PET/CT for sRT guidance might provide a possible benefit in this dedicated group of patients, which should be evaluated further. The currently ongoing, prospective PSMA-SRT study evaluates the success rate of sRT for recurrence of PCa after prostatectomy with and without planning based on PSMA-PET/CT [12], and this study includes patients with PSA > 0.1 ng/ml.
In this study, two clinical parameters (namely, seminal vesicle invasion in surgery and local recurrences on PET/CT imaging) had a significant impact on BR in multivariate analysis. Interestingly, local recurrent disease on PET/CT had a negative influence on BR (HR = 0.488). At initial observation, this finding is unexpected, as one would assume that macroscopic local disease could lead to an unfavorable BRFS. However, this might be interpreted by the fact that patients with such low PSA values and only local uptake in PET have a very low risk for distant metastatic disease. Thus, it is very likely that the recurrent prostate cancer in this patient group is cured by sRT to the prostatic fossa. Future studies should address the optimal sRT dose to the fossa [13] as well as to the local recurrence in PET/CT and whether admission of ADT is needed in this scenario [14]. Whether the used PSMA tracer has an impact on BRFS after sRT is not answered yet. In our study, patients with the 18F-PSMA-1007 tracer had a significant better detection rate compared to patients with the 68 Ga-PSMA-11 tracer. However, this was not translated into a significant better BRFS. Future studies with more patients and longer follow-up should assess this question. In addition, a study by Spohn et al. proposed that the SUVmax value with the local recurrent disease on PSMA-PET/CT images might be a predictive factor for BRFS after sRT [15].
Information on recurrence patterns on PSMA-PET/CT was available in 16 out of 60 patients with PSA relapse after sRT. Most patients had distant relapse and only one patient had recurrent disease inside the RT field, suggesting that implementation of PSMA-PET/CT information for sRT has a benefit in the local control of the disease.
Despite its multicenter character, our study has some limitations. First, due to its retrospective nature, PSMA-PET/CT/CT, sRT, and follow-up protocols were not consistent within all study centers. Moreover, central PET/CT imaging and pathology review, which ensures consistency between the different reports, has not been carried out. Third, the number of patients included in this study is relatively small as staging using PSMA-PET/CT is not frequently used in patients with PSA < 0.2 ng/ml. In addition, as the PSMA-PET/CT has been recently implemented in clinical practice, follow-up time in our study is relatively short. Finally, although our primary endpoint—biochemical recurrence—is commonly used in studies on sRT after surgery, it is not a surrogate endpoint for overall survival or death of prostate cancer [16].
Conclusion
This multicenter analysis included patients with PSMA-PET/CT-guided sRT at low PSA levels after primary prostatectomy (< 0.2 ng/ml). Our results demonstrate very promising BRFS rates as well as a low number of in-sRT field recurrences, suggesting that implementation of PSMA-PET/CT imaging for sRT guidance might be of benefit for this patient cohort. In addition, the presence of seminal vesicle invasion in surgery specimen and the absence of local recurrence in PET/CT were presented as new risk factors for BRFS after PSMA-guided sRT. Further prospective studies are warranted to validate our findings.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to GDPR reasons but are available from the corresponding author on reasonable request and under adherence to GDPR and ethic requirements.
References
Tourinho-Barbosa R, Srougi V, Nunes-Silva I, et al. Biochemical recurrence after radical prostatectomy: what does it mean? Int Braz J Urol. 2018;44:14–21. https://doi.org/10.1590/S1677-5538.IBJU.2016.0656.
Tendulkar RD, Agrawal S, Gao T, et al. Contemporary update of a multi-institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J Clin Oncol. 2016;34:3648–54. https://doi.org/10.1200/JCO.2016.67.9647.
Abugharib A, Jackson WC, Tumati V, et al. Very early salvage radiotherapy improves distant metastasis-free survival. J Urol. 2017;197:662–8. https://doi.org/10.1016/j.juro.2016.08.106.
Parker CC, Clarke NW, Cook AD, et al. Timing of radiotherapy after radical prostatectomy (RADICALS-RT): a randomised, controlled phase 3 trial. Lancet. 2020;396:1413–21. https://doi.org/10.1016/S0140-6736(20)31553-1.
Fendler WP, Calais J, Eiber M, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol. 2019;5:856–63. https://doi.org/10.1001/jamaoncol.2019.0096.
Cerci JJ, Fanti S, Lobato EE, et al. Diagnostic performance and clinical impact of (68)Ga-PSMA-11 PET/CT imaging in early relapsed prostate cancer after radical therapy: a prospective multicenter study (IAEA-PSMA Study). J Nucl Med. 2022;63:240–7. https://doi.org/10.2967/jnumed.120.261886.
Abghari-Gerst M, Armstrong WR, Nguyen K, et al. A comprehensive assessment of (68)Ga-PSMA-11 PET in biochemically recurrent prostate cancer: results from a prospective multicenter study on 2,005 patients. J Nucl Med. 2022;63:567–72. https://doi.org/10.2967/jnumed.121.262412.
Zamboglou C, Strouthos I, Sahlmann J, et al. Metastasis-free survival and patterns of distant metastatic disease after prostate-specific membrane antigen positron emission tomography (PSMA-PET/CT)-guided salvage radiation therapy in recurrent or persistent prostate cancer after prostatectomy. Int J Radiat Oncol Biol Phys. 2022;113:1015–24. https://doi.org/10.1016/j.ijrobp.2022.04.048.
Vogelius IR, Bentzen SM. Diminishing returns from ultrahypofractionated radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2020;107(2):299–304. https://doi.org/10.1016/j.ijrobp.2020.01.010.
Gupta SK, Watson T, Denham J, et al. Prostate-specific membrane antigen positron emission tomography-computed tomography for prostate cancer: distribution of disease and implications for radiation therapy planning. Int J Radiat Oncol Biol Phys. 2017;99:701–9. https://doi.org/10.1016/j.ijrobp.2017.06.2448.
De Visschere PJL, Standaert C, Futterer JJ, et al. A systematic review on the role of imaging in early recurrent prostate cancer. Eur Urol Oncol. 2019;2:47–76. https://doi.org/10.1016/j.euo.2018.09.010.
Calais J, Czernin J, Fendler WP, Elashoff D, Nickols NG. Randomized prospective phase III trial of (68)Ga-PSMA-11 PET/CT molecular imaging for prostate cancer salvage radiotherapy planning [PSMA-SRT]. BMC Cancer. 2019;19:18. https://doi.org/10.1186/s12885-018-5200-1.
Ghadjar P, Hayoz S, Bernhard J, et al. Dose-intensified versus conventional-dose salvage radiotherapy for biochemically recurrent prostate cancer after prostatectomy: the SAKK 09/10 Randomized Phase 3 Trial. Eur Urol. 2021;80:306–15. https://doi.org/10.1016/j.eururo.2021.05.033.
Carrie C, Magne N, Burban-Provost P, et al. Short-term androgen deprivation therapy combined with radiotherapy as salvage treatment after radical prostatectomy for prostate cancer (GETUG-AFU 16): a 112-month follow-up of a phase 3, randomised trial. Lancet Oncol. 2019;20:1740–9. https://doi.org/10.1016/S1470-2045(19)30486-3.
Spohn SKB, Farolfi A, Schandeler S, et al. The maximum standardized uptake value in patients with recurrent or persistent prostate cancer after radical prostatectomy and PSMA-PET/CT-guided salvage radiotherapy-a multicenter retrospective analysis. Eur J Nucl Med Mol Imaging. 2022;50:218–27. https://doi.org/10.1007/s00259-022-05931-5.
Jackson WC, Tang M, Schipper MJ, et al. Biochemical failure is not a surrogate end point for overall survival in recurrent prostate cancer: analysis of NRG Oncology/RTOG 9601. J Clin Oncol. 2022;40:3172–9. https://doi.org/10.1200/JCO.21.02741.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology—Genitourinary
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Solomonidou, N., Germanou, D., Strouthos, I. et al. PSMA-PET/CT-guided salvage radiotherapy in recurrent or persistent prostate cancer and PSA < 0.2 ng/ml. Eur J Nucl Med Mol Imaging 50, 2529–2536 (2023). https://doi.org/10.1007/s00259-023-06185-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06185-5