Abstract
Introduction
Alzheimer’s disease (AD) usually first presents in elderly patients, but may also develop at an earlier age. Patients with an early age at onset tend to present with complaints other than memory impairment, such as visuospatial problems or apraxia, which may reflect a different distribution of cortical involvement. In this study we set out to investigate whether age at onset in patients with AD determines the pattern of atrophy on cerebral MRI scans.
Methods
We examined 55 patients with AD over a wide age range and analyzed their 3-D T1-weighted structural MRI scans in standard space using voxel-based morphometry (VBM). Regression analysis was performed to estimate loss of grey matter as a function of age, corrected for mini-mental state examination (MMSE) scores and sex.
Results
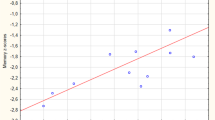
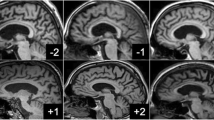
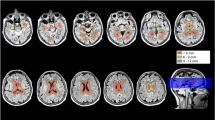
The VBM analyses identified multiple areas (including the temporal and parietal lobes), showing more atrophy with advancing age. By contrast, a younger age at onset was found to be associated with lower grey matter density in the precuneus. Regionalized volumetric analysis of this region confirmed the existence of disproportionate atrophy in the precuneus in patients with early-onset AD. Application of a multivariate model with precuneus grey matter density as input, showed that precuneal and hippocampal atrophy are independent from each other. Additionally, we found that a smaller precuneus is associated with impaired visuospatial functioning.
Conclusion
Our findings support the notion that age at onset modulates the distribution of cortical involvement, and that disproportionate precuneus atrophy is more prominent in patients with a younger age of onset.





Similar content being viewed by others
References
Galton CJ, Patterson K, Xuereb JH et al (2000) Atypical and typical presentations of Alzheimer’s disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain 123:484–498
Greicius MD, Geschwind MD, Miller BL (2002) Presenile dementia syndromes: an update on taxonomy and diagnosis. J Neurol Neurosurg Psychiatry 72:691–700
Ishii K, Kawachi T, Sasaki H et al (2005) Voxel-based morphometric comparison between early- and late-onset mild Alzheimer’s disease and assessment of diagnostic performance of z score images. AJNR Am J Neuroradiol 26:333–340
Frisoni GB, Testa C, Sabattoli F et al (2005) Structural correlates of early and late onset Alzheimer’s disease: voxel based morphometric study. J Neurol Neurosurg Psychiatry 76:112–114
Sakamoto S, Ishii K, Sasaki M et al (2002) Differences in cerebral metabolic impairment between early and late onset types of Alzheimer’s disease. J Neurol Sci 200:27–32
Scarmeas N, Habeck C, Anderson KE et al (2004) Altered PET functional brain responses in cognitively intact elderly persons at risk for Alzheimer disease (carriers of the epsilon4 allele). Am J Geriatr Psychiatry 12:596–605
Yasuno F, Imamura T, Hirono N et al (1998) Age at onset and regional cerebral glucose metabolism in Alzheimer’s disease. Dement Geriatr Cogn Disord 9:63–67
Cavanna AE, Trimble MR (2006) The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129:564–583
Jacobs D, Sano M, Marder K et al (1994) Age at onset of Alzheimer’s disease: relation to pattern of cognitive dysfunction and rate of decline. Neurology 44:1215–1220
Prvulovic D, Hubl D, Sack AT et al (2002) Functional imaging of visuospatial processing in Alzheimer’s disease. Neuroimage 17:1403–1414
Fujimori M, Imamura T, Yamashita H et al (1998) Age at onset and visuocognitive disturbances in Alzheimer disease. Alzheimer Dis Assoc Disord 12:163–166
McKhann G, Drachman D, Folstein M et al (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34:939–944
Qizilbash N (2002) Evidence-based dementia practice. Blackwell Science, Oxford, pp xxii, 893
Scheltens P, Leys D, Barkhof F et al (1992) Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry 55:967–972
Smith SM, Zhang Y, Jenkinson M et al (2002) Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 17:479–489
Chetelat G, Desgranges B, De La Sayette V et al (2002) Mapping gray matter loss with voxel-based morphometry in mild cognitive impairment. Neuroreport 13:1939–1943
Baron JC, Chetelat G, Desgranges B et al (2001) In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer’s disease. Neuroimage 14:298–309
Ashburner J, Friston KJ (2000) Voxel-based morphometry – the methods. Neuroimage 11:805–821
Woods RP (2003) Characterizing volume and surface deformations in an atlas framework: theory, applications, and implementation. Neuroimage 18:769–788
Freeborough PA, Fox NC (1998) Modeling brain deformations in Alzheimer disease by fluid registration of serial 3D MR images. J Comput Assist Tomogr 22:838–843
Hellier P, Ashburner J, Corouge I et al (2002) Intersubject registration of functional and anatomical data using SPM. LNCS 2489:590–597
Davatzikos C, Genc A, Xu D et al (2001) Voxel-based morphometry using the RAVENS maps: methods and validation using simulated longitudinal atrophy. Neuroimage 14:1361–1369
Brett M, Anton JL, Valabregue R et al (2002) Region of interest analysis using an SPM toolbox. 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan
Wechsler D (1997) Wechsler adult intelligence scale – third edition. The Psychological Corporation, San Antonio
Reitan RM (1958) Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills 8:271–276
Lindeboom J, Schmand B, Tulner L et al (2002) Visual association test to detect early dementia of the Alzheimer type. J Neurol Neurosurg Psychiatry 73:126–133
Luteijn F, Van der Ploeg FAE (1983) Groninger intelligentie test. Swets and Zeitlinger, Lisse
Luria AR (1966) Higher cortical functions in man. Basic Books, New York
Beery KE, Buktenica NA (1989) Developmental test of visual-motor integration. Psychological Assessment Resources, Odessa, FL
Shiino A, Watanabe T, Maeda K et al (2006) Four subgroups of Alzheimer’s disease based on patterns of atrophy using VBM and a unique pattern for early onset disease. Neuroimage 33:17–26
Imamura T, Takatsuki Y, Fujimori M et al (1998) Age at onset and language disturbances in Alzheimer’s disease. Neuropsychologia 36:945–949
Braak E, Griffing K, Arai K et al (1999) Neuropathology of Alzheimer’s disease: what is new since A. Alzheimer? Eur Arch Psychiatry Clin Neurosci 249(Suppl 3):14–22
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 82:239–259
Coleman PD, Flood DG (1987) Neuron numbers and dendritic extent in normal aging and Alzheimer’s disease. Neurobiol Aging 8:521–545
Scheff SW, Price DA (2001) Alzheimer’s disease-related synapse loss in the cingulate cortex. J Alzheimers Dis 3:495–505
Najlerahim A, Bowen DM (1988) Regional weight loss of the cerebral cortex and some subcortical nuclei in senile dementia of the Alzheimer type. Acta Neuropathol (Berl) 75:509–512
Bradley KM, O’Sullivan VT, Soper ND et al (2002) Cerebral perfusion SPET correlated with Braak pathological stage in Alzheimer’s disease. Brain 125:1772–1781
Wang L, Zang Y, He Y et al (2006) Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: evidence from resting state fMRI. Neuroimage 31:496–504
Burgess N, Maguire EA, Spiers HJ et al (2001) A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. Neuroimage 14:439–453
Maguire EA, Burgess N, Donnett JG et al (1998) Knowing where and getting there: a human navigation network. Science 280:921–924
Maguire EA, Frackowiak RS, Frith CD (1997) Recalling routes around London: activation of the right hippocampus in taxi drivers. J Neurosci 17:7103–7110
Mesulam MM, Nobre AC, Kim YH et al (2001) Heterogeneity of cingulate contributions to spatial attention. Neuroimage 13:1065–1072
Karnath HO, Perenin MT (2005) Cortical control of visually guided reaching: evidence from patients with optic ataxia. Cereb Cortex 15:1561–1569
Maddock RJ, Garrett AS, Buonocore MH (2001) Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience 104:667–676
Maguire EA (2001) The retrosplenial contribution to human navigation: a review of lesion and neuroimaging findings. Scand J Psychol 42:225–238
Shah NJ, Marshall JC, Zafiris O et al (2001) The neural correlates of person familiarity. A functional magnetic resonance imaging study with clinical implications. Brain 124:804–815
Bondi MW, Houston WS, Salmon DP et al (2003) Neuropsychological deficits associated with Alzheimer’s disease in the very-old: discrepancies in raw vs. standardized scores. J Int Neuropsychol Soc 9:783–795
Mai JK, Assheuer J, Paxinos G (2004) Atlas of the human brain. Academic Press, San Diego, p 246
Chau W, McIntosh AR (2005) The Talairach coordinate of a point in the MNI space: how to interpret it. Neuroimage 25:408–416
Acknowledgements
G. Karas is the recipient of Grant 2001-014 by the “Stichting Alzheimer Nederland” and S.A.R.B. Rombouts is the recipient of Grant H00.17 from “Hersenstichting Nederland”. The clinical database of the Alzheimer Center is supported by a grant from Stichting Dioraphte. Additional support was received from the Stichting Alzheimer & Neuropsychiatrie Foundation.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karas, G., Scheltens, P., Rombouts, S. et al. Precuneus atrophy in early-onset Alzheimer’s disease: a morphometric structural MRI study. Neuroradiology 49, 967–976 (2007). https://doi.org/10.1007/s00234-007-0269-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-007-0269-2