Abstract
In order to flexibly adapt to a permanently changing environment, it is necessary to inhibit previously activated but now irrelevant processing pathways. Empirically, this inhibition manifests itself only indirectly in terms of a cost of reengaging a previously inhibited pathway (n − 2 repetition costs). While imaging studies suggest an involvement of the prefrontal cortex in this type of inhibition, it has recently been argued that the underlying processes are implicated not in triggering inhibition, but in overcoming it. To disentangle these processes on a behavioral level, we investigated the time course of inhibition using a cued task switching paradigm. The response–cue interval (between the response of trial n − 1 and the cue of trial n) was varied in five steps to capture its influence on inhibition in a fine-grained manner. The results suggest that the impact of inhibitory processes increases during the first 200–300 ms after the response of the previous trial, reaches its full extent with about 300 ms, and starts to diminish after that. Therefore, future research on the neural correlates of n − 2 repetition costs should employ techniques with a high temporal resolution that are able to capture this presumed time course of inhibitory processes.
Similar content being viewed by others
Introduction
The ability to flexibly (re-)act in accordance with a permanently changing environment is a hallmark of human cognition. The task switching paradigm is widely used to investigate the basic processes that underlie this flexible adjustment. However, there is an ongoing discussion whether the so-called switch costs, one of the core measures in task switching, are due to active preparation processes in case of changing task demands, or whether inhibition of previously activated task sets enables switching to a different one (cf. Kiesel et al. 2010; Vandierendonck et al. 2010, for reviews). One phenomenon that seems to be especially useful to assess inhibitory processes in task switching is n − 2 repetition costs, first described by Mayr and Keele (2000). This effect refers to the observation that, when switching among three tasks A, B, and C, higher reaction times (RTs) and error rates (ERs) occur when the task that has to be performed in the current trial equals the task in trial n − 2 (i.e., sequences of type ABA) compared with two consecutive switches to another task (sequences CBA).
N − 2 repetition costs are assumed to reflect inhibition of the previously activated task set after a switch trial (i.e., in case of conflicting task demands) that persists for some time and, therefore, has to be overcome when the still inhibited task set has to become activated again (e.g., Koch et al. 2010). Although n − 2 repetition costs have been reported in many studies, it remains an open question when and how inhibition is triggered and how it develops over time. From a neural perspective (mainly based on studies using functional magnetic resonance imaging (fMRI)), there is convincing evidence that inhibition-related processes are located in the right prefrontal cortex (PFC), especially in the right inferior frontal gyrus (e.g., Aron et al. 2004a, b; Dreher and Berman 2002; Mayr et al. 2006). As indicated by Dreher and Berman (2002), the mentioned studies are not able to distinguish whether these brain areas are involved in triggering inhibitory processes or whether they are engaged in overcoming residual inhibition. A recent study conducted by Whitmer and Banich (2012) suggests that the PFC is indeed merely involved in overcoming inhibition. Regressing individual differences in the size of n − 2 repetition costs onto brain activity during task switching, they found that activity of the putamen and the supplementary motor area and premotor cortex is related to stronger inhibition of previous task sets. The authors conclude that these brain areas play a role in inhibition. The exact functions of these regions for inhibition, however, remain unclear.
Until now, there is a current debate regarding the mechanism underlying n − 2 repetition costs. The two main accounts will be briefly discussed here (see Grange et al. 2013, for a direct comparison).
On the one hand, a self-inhibitory mechanism is proposed that causes the task just executed to inhibit itself. For instance, Houghton and Tipper (1994, 1996) introduced a model of inhibition in selective attention including feedback loops that cause objects to inhibit themselves. A more recent model assuming self-inhibition as the basic mechanism underlying n − 2 repetition costs was proposed by Grange et al. (2013). Contrary to the model of Houghton and Tipper (1994, 1996), it is assumed that it is not an external stimulus triggering the onset of inhibition, but that each task is inhibited immediately after its use, at least under conditions in which frequent switches are required. Therefore, inhibition is supposed to start at the end of trial n − 2 and to slightly decay after that.
On the other hand, some authors favor a mechanism of lateral inhibition by which the inhibition of the previous task is a by-product of activating the current task in case of a task switch (Gade and Koch 2005; Philipp and Koch 2006). As a trigger for inhibition, a task- or response conflict caused by multivalent stimuli associated with different tasks is assumed. This conflict needs to be solved by inhibition. A broader discussion of the two proposed inhibitory mechanisms is deferred to the section “General Discussion”.
One obstacle for a more comprehensive understanding of the component processes underlying the phenomenon of n − 2 repetition costs is a lack of knowledge regarding its time course. It has been argued that inhibition decays gradually over time in a passive manner, focusing mainly on the finding of n − 2 repetition cost being independent of the length of the preparation interval in the majority of experiments (cf. Koch et al. 2010). However, recent findings doubt this assumption. Grange and Houghton (2011) analyzed RT distributions and provide evidence for the possibility of overcoming inhibition if sufficient preparation has taken place. In addition, studies focusing on preparation time of trials n − 1 and n − 2 (Scheil and Kleinsorge 2014) or taking into account the amount of information provided by the cue (Gade and Koch 2014) report effects of preparation on n − 2 repetition costs (albeit the influence of the effects of preparation time remain ambiguous).
A possibility to disentangle processes involved in triggering inhibition or in overcoming it lies in the variation of different time intervals in the cued task switching paradigm. The cue–stimulus interval (CSI) allows participants to prepare for the next task. Consequently, effects of the CSI can be supposed to reflect active processes. On the other hand, no specific preparation for the next task is possible during the response–cue interval (RCI), that is, the interval between the response of trial n − 1 and the cue of trial n, in which usually a fixation mark is presented that contains no information about the next task. Therefore, effects of the RCI cover passive processes, such as decaying inhibition or decaying activation.
While the CSI has been considered in many studies focusing on n − 2 repetition costs, the RCI, offering the possibility of investigating effects of decay processes independent of preparation, has been studied less. Most studies varied the RCI inversely to the CSI to ensure a constant response–stimulus interval (RSI), therefore confounding the RCI with the possibility to prepare for the next task (e.g., Bao et al. 2006, Exp. 3a; Koch et al. 2004, Exp. 1; Philipp and Koch 2006, Exp. 1; Philipp et al. 2007, Schuch and Koch 2003). In the few studies allowing a differentiation between the effects of RCI and CSI, the results are rather mixed. While some experiments suggest no influence of the RCI (Mayr 2002) or only of the RCI after trial n − 2 (Gade and Koch 2005), other studies found a decrease in n − 2 repetition costs with increasing RCI (Grange and Houghton 2009; Koch et al. 2004, Exp. 2; Mayr and Keele 2000, Exp. 1a and 1b). Some authors argue that this decrease is due to weaker persisting activation of the task executed in the previous trial for longer RCIs, leading to a reduced amount of interference and, therefore, to a reduced need for inhibition (e.g., Gade and Koch 2005; Koch et al. 2010). Others (e.g., Grange et al. 2013) assume a passive dissipation of inhibition over time. Recently, Vandierendonck (2013) raised doubts concerning the assumption of decaying inhibition but proposed that strategic choices or temporal distinctiveness is responsible for n − 2 repetition costs. Based on the diverging findings regarding the effect of the RCI on n − 2 repetition costs, it is not possible to distinguish between those accounts.
The divergent findings concerning the time course of inhibition may to some degree be due to different lengths of the RCI used in the experiments. All of the mentioned studies varied the RCI in two steps (short/long), with large differences regarding the temporal interval between the implemented RCIs. These range from RCIs of 50 ms and 550 ms in Experiment 1a of Mayr and Keele (2000), thus creating an absolute difference of 500 ms between long and short RCI, to 100 and 1,900 ms in Experiment 2 of Gade and Koch (2005), leading to a time gap of 1,800 ms. Therefore, the intervals between short and long RCIs differ largely between studies and are thus not easily comparable. Besides, the levels of the RCI that have been used so far are temporally very distinct from each other, being separated by at least 500 ms. Consequently, possible nonlinear trends concerning the strength of inhibition during this gap (which amounts to at least half a second) cannot be captured.
The present study aimed at investigating the time course of inhibition in a more fine-grained manner. For this purpose, the length of the RCI was manipulated in five steps separated by 125 ms each. This allowed us to trace the time course of inhibition in a fine-grained manner, being able to capture also nonlinear trends of the size of n − 2 repetition costs that could be overlooked in designs using only two distinct RCIs.
Regarding the expected time course of n − 2 repetition costs, different predictions can be derived from the different theoretical approaches. On the one hand, based on accounts assuming passive decay of inhibition, one would expect highest n − 2 repetition costs for shortest RCIs that decrease when the RCI is prolonged. The same holds for accounts supposing persisting activation of the previous task to trigger inhibition. On the other hand, if one assumes active processes involved in inhibition that take time to build up, n − 2 repetition costs should be small at short RCIs, when inhibition is not yet fully established. If both aspects, active setup of inhibition and decay processes, play a role, n − 2 repetition costs should be highest at RCIs of medium length, when there was enough time for inhibition to build up and the influence of decay processes is still small.
Experiment I
Method
Participants
Thirty-one subjects (10 male) with a mean age of 23.8 years (range: 19–30) participated. All had normal or corrected-to-normal vision. One additional participant was excluded from further analyses due to more than 35 % errors.
Stimuli, tasks, and apparatus
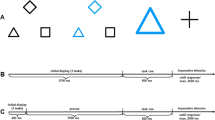
Imperative stimuli consisted of digits from range 1 to 9 (excluding 5). Each digit was about 7 mm in height and 4 mm in width. Task precues consisted of a dark blue square, diamond, or triangle surrounding the position of the imperative stimulus with a size of about 7 cm × 7 cm. There were three numerical judgment tasks, one regarding the magnitude (smaller vs. larger than five, indicated by the diamond), one regarding the parity of the digits (odd or even, indicated by the square), and a third regarding the position of the digit along the number line relative to the digit 5 (1, 2, 8, and 9 were considered as peripheral digits, 3, 4, 6, and 7 as central ones). This task was indicated by the triangle.
Stimuli were presented centrally on a 17″ monitor in black on light gray background. Viewing distance was not restricted but amounted to approximately 60 cm. Half of the participants pressed the ‘y’-key of a German QWERTZ keyboard for small, even, and central digits and the ‘−’-key for large, odd, and peripheral ones. For the other participants, these mappings were reversed.
Design and procedure
The experimental design resulted from a factorial combination of the within-subjects factors RCI, varied in five steps across trials (50, 175, 300, 425, and 550 ms), and task sequence (ABA vs. CBA). Task repetitions were not allowed.
The experiment was run in a single session that took about 60 min. It consisted of 12 blocks of 120 trials each. Each combination of task and RCI occurred with equal frequency in a pseudo-random order.
The present study was carried out conforming to the principles laid down by the Helsinki Declaration. After giving informed consent, participants were provided with on-screen instructions in which the tasks and the meaning of the task cues were explained. Instructions emphasized speed as well as accuracy.
Each trial began with the presentation of a fixation mark for the duration of the RCI, followed by the task cue. The CSI was held constant at 600 ms. After that, the imperative stimulus was presented and remained visible together with the task cue until the participant’s response. In case of an error, error feedback was presented for additional 1,000 ms; in case of RTs higher than the RT deadline of 2,500 ms, RT feedback was presented for additional 1,000 ms.
Results
The first block was considered as practice and therefore excluded from analyses. The first three trials of each block were omitted, as were trials with RTs exceeding 2,500 ms (.6 %). In addition, trial triplets in which an error occurred in trials n − 1 or n − 2 were excluded (11.2 %). From RT analysis, errors in the current trial were also excluded (5.1 %). Mean individual RTs and ERs were subjected to analyses of variance with the within-subjects factors RCI (50, 175, 300, 425, or 550 ms) and task sequence (ABA vs. CBA).
For RT data, significant n − 2 repetition costs of 29 ms occurred, F (1, 30) = 48.57, p < .001, \( \eta_{p}^{2} \) = .62. This effect was modulated by the RCI, F (4, 120) = 3.30, p < .05, \( \eta_{p}^{2} \) = .10 (cf. Table 1). As established by Duncan’s post hoc tests, n − 2 repetition costs were significant only for RCIs of length 300, 425, and 550 ms (p’s < .05), while for the other conditions, only numerical n − 2 repetition costs occurred (p’s > .12, see Fig. 1). Direct comparisons revealed largest n − 2 repetition costs for RCIs of length 300 ms that differed significantly (p‘s < .05) from those for RCIs of length 50, 175, and 425 ms. The difference to n − 2 repetition costs for RCIs of 550 ms failed to reach significance (p > .14), as did all other pairwise comparisons. Besides, the main effect of RCI turned out to be significant, F (4, 120) = 2.74, p < .05, \( \eta_{p}^{2} \) = .08. This effect was Greenhouse–Geisser corrected (ε = .83). For RCIs of 50 ms, mean RTs were significantly larger (757 ms) than for RCIs of 425 (738 ms) or 550 ms (741 ms, p’s < .05), while for RTs of the RCIs of 175 ms (745 ms) and 300 ms (747 ms), no significant differences occurred.
For ER data, neither the main effects nor their interaction reached statistical significance (p’s > .22).
Discussion
In Experiment I, n − 2 repetition costs were significantly modulated by the length of the RCI. While for RCIs of 50 ms and 175 ms, only numerical costs occurred, they were much larger for longer RCIs. Reaching their maximum at 300 ms, costs started to diminish when the RCI was longer. However, the finding of highest n − 2 repetition costs at RCIs of 300 ms has to be replicated to prove the stability of the data pattern. Moreover, a slight increase in n − 2 repetition costs occurred for RCIs of 550 ms, although a further decrease was expected. This increase was small but needs further elaboration to rule out the possibility of a systematic increase. Therefore, a second experiment was conducted to replicate the findings of Experiment I. Three different tasks were used to examine whether the results can be generalized to different experimental designs.
Experiment II
Method
Participants
Twenty-one new subjects (6 male) with normal or corrected-to-normal vision participated. Their mean age was 22.7 years (range: 19−26). One additional participant was excluded from further analyses due to more than 40 % errors. Using G*Power (Faul et al. 2007), a sample size of 20 participants was determined a priori to be sufficient to replicate the critical interaction between task sequence and RCI.
Stimuli, tasks, and apparatus
Stimuli consisted of two different shapes (× and +) presented in yellow or blue and with a size of either 3 cm × 3 cm or 6 cm × 6 cm. Task cues consisted of a dark gray diamond, square, or triangle surrounding the position of the imperative stimulus with a size of about 7 cm × 7 cm. Participants switched among three perceptual decision tasks in which they had to judge the stimuli regarding their size (big vs. small, indicated by the diamond), color (yellow vs. blue, indicated by the square), or their shape (× vs. +, indicated by the triangle). Stimuli were presented centrally on a light gray background. Viewing distance was not controlled but approximated 60 cm. Half of the participants pressed the ‘y’-key of a German QWERTZ keyboard for small, blue, and x-shaped stimuli and the ‘−’-key for big, yellow, and +-shaped stimuli. For the other half of the sample, the response mappings were reversed.
Design and procedure
These were the same as in Experiment I. The only difference was that task cues disappeared with target onset in order to facilitate the perceptual judgment of the shapes.
Results
The first block was considered as practice and excluded from analyses, as were the first three trials of each block. Due to fatigue effects, the last block was excluded as well. In addition, trials with RTs exceeding 2,500 ms were omitted (1.1 %), as were trial triplets involving an error in trial n − 1 or n − 2 (11.8 %). From RT analyses, error trials were also excluded (5.0 %).
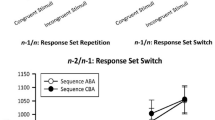
For RT data, significant n − 2 repetition costs of 48 ms occurred, F (1, 20) = 48.25, p < .001, \( \eta_{p}^{2} \) = .71. Besides, there was a main effect of RCI, F (4, 80) = 2.87, p < .05, \( \eta_{p}^{2} \) = .13. Duncan’s post hoc tests revealed that this effect was due to RTs for the shortest RCI (839 ms) being significantly higher (p’s < .05) compared with RTs for RCIs of length 300 (817 ms), 425 (816 ms), and 550 (810 ms), with no difference compared with RCI 175 (824 ms). Importantly, the interaction of both factors reached statistical significance, F (4, 80) = 2.86, p < .05, \( \eta_{p}^{2} \) = .12. Significant n − 2 repetition costs occurred for all RCIs (p’s < .05). Duncan’s post hoc tests revealed highest costs for the RCI of 300 ms that differed significantly (p’s < .05) from n − 2 repetition costs for RCIs of length 50, 175, and 550 ms but not from those for the RCI of 425 ms (p > .31, see Fig. 2).
For ER data, marginal significant n − 2 repetition costs of .6 % occurred, F (1, 20) = 3.46, p < .08, \( \eta_{p}^{2} \) = .15. Neither the main effect of RCI nor its interaction with task sequence reached statistical significance (p’s > .26), though the data pattern of the interaction mirrored RT data (Table 2).
Combined analysis of Experiments I and II
In an additional analysis, we combined the data of both experiments and reran the RT ANOVA using a more conservative post hoc test.
Overall, significant n − 2 repetition costs of 36 ms occurred, F (1, 51) = 87.46, p < .001, \( \eta_{p}^{2} \) = .63. This effect significantly differed between the two experiments, F (1, 51) = 6.38, p < .05, \( \eta_{p}^{2} \) = .06, due to higher n − 2 repetition costs in Experiment II (48 ms) compared with Experiment I (29 ms). The main effect of RCI was significant as well, F (4, 204) = 5.31, p < .001, \( \eta_{p}^{2} \) = .09. RTs for the shortest RCI were significantly higher (790 ms) compared with RTs for RCIs of length 300 (775 ms), 425 (769 ms), and 550 (768 ms) and marginally (p < .09) higher than RTs for the RCI of length 175 (776 ms). The interaction of both factors reached statistical significance, F (4, 204) = 5.49, p < .001, \( \eta_{p}^{2} \) = .10. Tukey’s post hoc test revealed marginally significant (p < .08) n − 2 repetition costs for the shortest RCI; for the other RCIs, these costs were significant (p’s < .05). N − 2 repetition costs were largest for the RCI of 300 ms (63 ms) and differed significantly (p’s < .05) from the costs occurring at RCIs of 50 (22 ms), 175 (23 ms), and 550 ms (35 ms), but not at 425 ms (40 ms, p > .15).
Discussion
Experiment II nicely replicated the findings of Experiment I. Again, the size of n − 2 repetition costs increased from RCIs of length 50 to 300 ms, where highest costs emerged. However, in contrast to Experiment I, no further increase was visible from 425 to 550 ms. The same holds for the joint analysis of the data from both experiments. Therefore, a systematic increase in n − 2 repetition costs from 425 to 550 ms seems to be unlikely. Instead, Experiment II as well as the joint analysis suggests a decrease in n − 2 repetition costs when the RCI is longer than about 300 ms.
However, models like that of Grange et al. (2013) argue that inhibition starts at the end of trial n − 2. If this was the case, the current RCI would be a very indirect measure of the time course of n − 2 repetition costs. Therefore, and to make the present study comparable with that of Gade and Koch (2005), the RCI between the response of trial n − 2 and the cue of trial n − 1 (in the following termed lag_RCI) was included in additional analyses of the data of both experiments. This further allows looking for possible sequential effects of the time intervals. These analyses yielded a quite unsystematic picture, but highest n − 2 repetition costs still occurred when the RCI between the response of trial n − 1 and the cue of trial n had a length of 300 ms, being in line with the main finding of Experiments I and II. Furthermore, when adding lag_RCI and the current RCI to yield a clearer view of their effects, n − 2 repetition costs increased from RCIs summing up to 100 ms to RCIs summing up to 850 ms, where they reached their maximum before decreasing again. The complete analyses are provided in the appendix.
General discussion
The present study investigated the time course of inhibition in task switching. For this purpose, the RCI was varied in five steps, and its influence on n − 2 repetition costs was examined. While significant n − 2 repetition costs could be observed for RCIs of 300, 425, and 550 ms, they were smaller and partly insignificant when the RCI was shorter (50 or 175 ms). Furthermore, the size of n − 2 repetition costs declined again when the RCI exceeded 300 ms, that is, with an RCI of 425 and 550 ms. In addition, the analysis in terms of the sum of RCI and lag_RCI yielded an increase in n − 2 repetition costs for summed RCIs from 100 to 850 ms, where the maximum could be observed. Furthermore, a significant decrease for longer intervals (975 and 1,100 ms) was visible. This pattern suggests a nonlinear development of inhibition over time. On the one hand, inhibition seems to need about 200–300 ms, estimated from the current RCI alone, or about 2,800–2,900 ms, estimated from the end of trial n − 2 until the response of trial n, to reach its full extent, reflecting a process of building up a sufficient amount of inhibition in order to cope with interference of recently established task sets. Importantly, while there have been investigations focusing on how and when the impact of inhibitory processes declines (e.g., via passive decay or dependent on prior task conflict, cf. Koch et al. 2010), the present study is the first trying to identify the time needed for inhibition to reach its full extent. These observations can be taken as an indication for some overshoot of inhibition because the maximal inhibitory effect seems to occur when the goal of inhibition (the selection of a previously competing task) has already been accomplished (cf. Houghton and Tipper 1994, for a similar argument).
On the other hand, our results suggest that n − 2 repetition costs, after reaching their maximum with an RCI of about 300 ms, start to decline. Therefore, the results are in line with the assumption of inhibition that, once exerted, decreases during the RCI. However, based on the present data, it is not possible to distinguish whether this decrease happens in a passive manner or depends on prior task activation. As will be outlined below, we suggest that the amount of inhibition depends on the activation of the task to be inhibited (cf. Gade and Koch 2005; Koch et al. 2010).
For both experiments, not only the current RCI but also a combination of the current RCI and lag_RCI had an influence on the size of n − 2 repetition costs. This is in line with the assumption of Grange et al. (2013) that inhibition starts immediately after the response in trial n − 2. However, there are two possible interpretations of the present results. First, and in line with Grange et al. (2013), it could be argued that inhibition manifests itself during the whole time interval between the end of trial n − 2 and the response of trial n and that all timeframes lying in between contribute to the size of n − 2 repetition costs. Note that this would imply an interval of up to 3 s under the present circumstances because not only two RCIs and CSIs but in addition the RT of trial n − 1 would enter into this time window. Therefore, this estimation can only be approximative, as noise is added to this time interval by the participant’s RTs. In addition, many different processes can take place during 3 s in a cognitive experiment. Hence, no strong conclusions should be drawn based on this estimation. Second, it is possible that the lag_RCI in the present study affected n − 2 repetition costs rather erratically and that it is only the current RCI that influences inhibition. Based on the present data, it is not possible to distinguish between these two accounts. In either case, the implication for future studies would be to carefully control for temporal effects of every time interval between trials n − 2 and n before drawing conclusions regarding their influence on inhibitory processes.
For both experiments, RCIs of the same length were used that were equally spaced, with the critical RCI of 300 ms being in the middle position. Hence, it is possible that strategic choices led participants to trigger inhibition with a peak at 300 ms. To examine this possibility, one would need to disentangle the serial position of the RCI from its absolute length. In addition, no conclusions can be drawn regarding what exactly is inhibited. Nonetheless, the results at hand add important knowledge about the time course of n − 2 repetition costs. Nevertheless, further research is needed to shed light upon this issue.
Concerning the question whether self-inhibition or lateral inhibition causes n − 2 repetition costs, Grange et al. (2013) argue that the basis for this effect must be of self-inhibitory nature because in the case of lateral inhibition, the spreading inhibition should suppress both competing tasks in each trial. To follow the argumentation of Grange et al. (2013), if this was the case, n − 2 benefits instead of costs should be observed because in a sequence of type CBA, task A was inhibited twice (when task C was executed in trial n − 2 and again when task B was executed in trial n − 1), whereas for ABA sequences, task A was activated in trial n − 2 and inhibited only once in trial n − 1. However, this argument presupposes that lateral inhibition is applied to the same degree across all competing tasks irrespective of their respective activation. As has been shown recently by a study from our laboratory (Scheil and Kleinsorge 2014), the amount of n − 2 repetition costs is yet affected by the CSIs of trials n − 1 and n − 2. This strongly suggests that inhibition, whether lateral inhibition or self-inhibition, is applied in a flexible manner depending on the need for inhibition as a function of the activation of what is to be inhibited. Thus, the present results could be explained by self-inhibition as well as lateral inhibition if one additionally assumes that inhibition (i.e., its amount and, for the lateral inhibition account, also its direction toward the other tasks) depends on the prior activation of the to-be-inhibited task.
The supposition of task activation having an influence on n − 2 repetition costs leads to the study of Gade and Koch (2005) who tried to disentangle decaying task activation and decaying task inhibition. In their experiment, the RCI before trial n − 1 influenced n − 2 repetition costs, but the RCI before trial n had no impact. Based on these results, the authors doubt the assumption of decaying inhibition but relate n − 2 repetition costs to task conflict in trial n − 1 that loses its impact with decaying task activation. Based on the present results as well as on the results of Scheil and Kleinsorge (2014), we favor the assumption of task inhibition depending on prior task activation; therefore, we argue that both factors are involved in n − 2 repetition costs.
However, apart from the theoretical implications, the data pattern of the present study seems to be at odds with that one of Gade and Koch (2005), because we observed an interaction of the task sequence and the RCI between the response of trial n − 1 and the cue of trial n but no influence of the RCI between the response of trial n − 2 and the cue of trial n − 1. The opposite pattern occurred in the experiments of Gade and Koch. Because Gade and Koch (2005) used RCIs very distinct from each other (100 ms and 1,400 ms in Exp. 1 and 100 ms and 1,900 ms in Exp. 2), it is possible that influences of the RCI manifesting itself somewhere within the time span between the RCIs could not be detected. In addition, Gade and Koch used a CSI of 100 ms, therefore providing participants with a limited amount of time to prepare for the next task. In contrast, we used a CSI of 600 ms which can be supposed to be long enough for advanced preparation to take place. Therefore, differences regarding the impact of time intervals between the present study and that one of Gade and Koch can be related to methodological differences.
Importantly, all levels of the RCI covered in the present study would have been subsumed under the label “short RCI” in previous studies using only two levels of RCI. Of course, our considerations regarding the time course of inhibition are not meant to imply that the dynamics of inhibitory processes are invariant across different types of tasks or experimental paradigms, but this emphasizes the importance of investigating the time course of inhibition in a temporarily fine-grained manner in order to be able to detect its relatively subtle dynamics.
It would be interesting to further support the notion of inhibition reaching its full extent at about 200–300 ms after the response using event-related potentials (ERPs). To our best knowledge, the only study investigating n − 2 repetition costs together with response-locked ERPs was conducted by Sinai et al. (2007). They found effects of the task sequence (ABA vs. CBA) in cue-locked as well as in response-locked ERPs, concluding that inhibition is associated with both task and response preparation. Unfortunately, the RCI was blocked at 200 ms in this study, allowing no detailed analyses of RCI length and, therefore, the time needed to trigger inhibition.
Irrespective of the precise mechanisms involved, our observations strongly argue for a highly dynamic, nonlinear time course of inhibitory processes. Thus, given the low temporal resolution of the fMRI technique, its potential in disentangling the role of different brain areas, such as the PFC or basal ganglia, in inhibition seems to be limited. Using imaging methods with a higher temporal resolution, such as magnetoencephalography (MEG), may prove to be a more fruitful avenue for shedding additional light upon this issue.
To conclude, the present study investigated the time course of inhibition in task switching using a fine-grained variation of the length of the RCI. The results favor the assumption of n − 2 repetition costs being due to inhibitory processes that, as measured from the onset of the response in trial n − 1, need about 200–300 ms to be fully established. After reaching their main impact at about 300 ms after the response in the previous trial, they start to decay. Future research focusing in neural correlates of inhibitory processes should take this time course into account.
References
Aron AR, Monsell S, Sahakian BJ, Robbins TW (2004a) A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain 127:1561–1573
Aron AR, Robbins TW, Poldrack RA (2004b) Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8:170–177
Bao M, Li Z-H, Chen X-C, Zhang DR (2006) Backward inhibition in a task of switching attention within verbal working memory. Brain Res Bull 69:214–221
Dreher J-C, Berman KF (2002) Fractionating the neural substrate of cognitive control processes. P Natl Acad Sci USA 99:14595–14600
Faul F, Erdfelder E, Lang AG, Buchner A (2007) G* power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191
Gade M, Koch I (2005) Linking inhibition to activation in the control of task sequences. Psychon B Rev 12:530–534
Gade M, Koch I (2014) Cue type affects preparatory influences on task inhibition. Acta Psychol 148:12–18
Grange JA, Houghton G (2009) Temporal cue-target overlap is not essential for backward inhibition in task switching. Q J Exp Psychol 62:1068–1079
Grange JA, Houghton G (2011) Task preparation and task inhibition: a comment on Koch, Gade, Schuch, & Philipp (2010). Psychon Bull Rev 18:211–216
Grange JA, Juvina I, Houghton G (2013) On costs and benefits of n − 2 repetitions in task switching: towards a behavioural marker of cognitive inhibition. Psychol Res 77:211–222
Houghton G, Tipper SP (1994) A model of inhibitory mechanisms in selective attention. In: Dagenbach D, Carr TH (eds) Inhibitory processes in attention, memory, and language. Academic Press, San Diego, CA, pp 35–112
Houghton G, Tipper SP (1996) Inhibitory mechanisms of neural and cognitive control: applications to selective attention and sequential action. Brain Cognition 30:20–43
Kiesel A, Steinhauser M, Wendt M, Falkenstein M, Jost K, Philipp AM, Koch I (2010) Control and interference in task switching—a review. Psychol Bull 136:849–874
Koch I, Gade M, Philipp AM (2004) Inhibition of response mode in task switching. Exp Psychol 51:52–58
Koch I, Gade M, Schuch S, Philipp AM (2010) The role of inhibition in task switching: a review. Psychon B Rev 17:1–14
Los SA, Van Den Heuvel EC (2001) Intentional and unintentional contributions to nonspecific preparation during reaction time forperiods. J Exp Psychol Human 27:370–386
Mayr U (2002) Inhibition of action rules. Psychon B Rev 9:93–99
Mayr U, Keele SW (2000) Changing internal constraints on action: the role of backward inhibition. J Exp Psychol Gen 129:4–26
Mayr U, Diedrichsen J, Ivry R, Keele SW (2006) Dissociating task-set selection from task-set inhibition in the prefrontal cortex. J Cogn Neurosci 18:1–8
Philipp AM, Koch I (2006) Task inhibition and task repetition in task switching. Eur J Cogn Psychol 18:624–639
Philipp AM, Gade M, Koch I (2007) Inhibitory processes in language switching? Evidence from switching language-defined response sets. Eur J Cogn Psychol 19:395–416
Scheil J, Kleinsorge T (2014) N − 2 repetition costs depend on preparation in trials n − 1 and n − 2. J Exp Psychol Learn 40:865–872
Schuch S, Koch I (2003) The role of response selection for inhibition of task sets in task shifting. J Exp Psychol Human 29:92–105
Sinai M, Goffaux P, Phillips NA (2007) Cue- versus response-locked processes in backward inhibition: evidence from ERPs. Psychophysiology 44:596–609
Vandierendonck A (2013) On the role of dissipating inhibition in task switching. Z Psychol 221:61–62
Vandierendonck A, Liefooghe B, Verbruggen F (2010) Task switching: interplay of reconfiguration and interference control. Psychol Bull 136:601–626
Whitmer AJ, Banich MT (2012) Brain activity related to the ability to inhibit previous task sets: an fMRI study. Cogn Affect Behav Neurosci 12:661–670
Acknowledgments
We thank Miriam Gade and Jim Grange for their helpful comments on a previous version of this article and Florian Selchow for running the experiments.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
The procedures employed in the present study were approved by the ethics committee of the Leibniz Research Centre for Working Environment and Human Factors.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Supplementary analyses
RT and ER data of both experiments were analyzed with the additional factor lag_RCI (i.e., the time interval between the response of trial n − 2 and the cue of trial n − 1). This resulted in a 5 (RCI) × 5 (lag_RCI) × 2 (task sequence) ANOVA. Only results involving the lag_RCI factor are reported.
Experiment I
For RT data, the interaction between RCI and lag_RCI reached statistical significance, F (16, 480) = 3.41, p < .001, \( \eta_{p}^{2} \) = .10. This effect was mainly due to shortest RTs for conditions with an RCI of 550 ms and a lag_RCI of 50 ms (703 ms) as well as highest RTs for the RCI 50 ms/lag_RCI 550 ms condition (781 ms). In addition, the three-way interaction between RCI, lag_RCI, and task sequence was significant, F (16, 480) = 2.82, p < .001, \( \eta_{p}^{2} \) = .09. This interaction mainly resulted from highest n − 2 repetition costs in the conditions with a current RCI of 300 ms and a lag_RCI of either 300 ms (97 ms) or 550 ms (98 ms). An overview of the data is given in Table 3. Note that due to 50 conditions resulting from the present design, net n − 2 repetition costs are presented to reduce the table to 25 cells.
For ER data, neither of the effects reached statistical significance.
Experiment II
For RT data, a significant main effect of lag_RCI occurred, F (4, 80) = 2.74, p < .05, \( \eta_{p}^{2} \) = .12. Duncan’s post hoc test revealed shortest RTs for lag_RCIs of 50 ms (802 ms) that differed significantly (p’s < .05) from RTs for lag_RCIs of length 300 (823 ms), 425 (832 ms), and 550 (826 ms) but not from lag_RCI 175 (817 ms, p > .12). Besides, the interaction between RCI and lag_RCI was significant, F (16, 320) = 2.28, p < .01, \( \eta_{p}^{2} \) = .10. Shortest RTs emerged for conditions with a lag_RCI of 50 ms and an RCI of 175 ms (807 ms). Highest RTs could be observed for conditions with a lag_RCI of 300 ms and a RCI of 50 ms (908 ms) as well as for conditions with a lag_RCI of 550 ms and a RCI of 175 ms (892 ms). Additionally, the three-way interaction of all factors reached statistical significance, F (16, 320) = 2.69, p < .001, \( \eta_{p}^{2} \) = .12. This interaction was mainly caused by highest n − 2 repetition costs for conditions with a current RCI of 300 ms and a lag_RCI of 300 (139 ms), 425 (103 ms), or 550 ms (120 ms). An overview of the data is given in Table 4. Note that, as for Experiment I, net n − 2 repetition costs are presented to enhance readability.
For ER data, there was a significant interaction of RCI and lag_RCI, F (16, 320) = 1.83, p < .05, \( \eta_{p}^{2} \) = .08. This effect was mainly due to lowest ERs when both RCI and lag_RCI had a length of 50 ms (2.6 %) and highest ERs with a RCI of 175 ms and a lag_RCI of 550 ms (6.5 %), with the other conditions lying in between (cf. Table 4).
Please note that the two-way interaction between the current RCI and task sequence was still significant for RT data of both experiments (both p’s < .01), whereas the two-way interaction of lag_RCI and task sequence was far from significance (p > .61 and p > .48, respectively).
Due to the large number of different conditions, the results of the supplementary analyses provide a rather unclear picture for both experiments. To include the current RCI as well as lag_RCI in the analyses in a more transparent way, we used the sum of lag_RCI and RCI as factor in an additional step. This resulted in a new summed RCI variable involving nine different time intervals (100, 225, 350, 475, 600, 725, 850, 957, and 1,100 ms) that was included as within-subjects factor in analyses of variance, conducted for RT and ER net n − 2 repetition costs across the data of both experiments.
As can be seen from Fig. 3, the sum RCI significantly influenced the size of RT n − 2 repetition costs, F (8, 408) = 4.22, p < .001, \( \eta_{p}^{2} \) = .08. As established by Tukey’s post hoc tests, this effect was due to highest costs for conditions with lag_RCI and RCI summing up to 850 ms that differed significantly from conditions with 225, 475, 975, and 1,100 ms (p’s < .05) and marginally significant (p < .10) from the 350 ms condition.
For ER data, the effect of the summed RCI factor on n − 2 repetition costs was not significant (p > .57).
Discussion
To complete data analyses, additional ANOVAs including the RCI between the response of trial n − 2 and the cue of trial n − 1 were run. For both experiments, an interaction between lag_RCI and RCI occurred. Highest RTs emerged in conditions in which the current RCI was shorter than the lag_RCI, while shortest RTs occurred for RCIs longer than lag_RCI. This data pattern is in line with the trace conditioning account put forward by Los and Van Den Heuvel (2001) that proposes higher RTs when the lag_RCI is longer than the current RCI compared with conditions when the lag_RCI is shorter or equal to the current RCI. However, for the present data, the trace conditioning account holds only for the minimum and maximum RTs, while the other conditions lying in between show no systematic variation that could be explained by the model.
The three-way interaction of lag_RCI, RCI, and task sequence yielded a quite unsystematic picture, although highest n − 2 repetition costs still occurred with a current RCI of 300 ms, as it was the case for the two-way interaction. However, using the sum of both time intervals, the data pattern was much more clear-cut, implying highest n − 2 repetition costs when both RCIs summed up to 850 ms. Considering all time intervals lying between the response of trial n − 2 and the response of the current trial (both RCIs, CSIs, and a mean RT of 775 ms), this leads to the observation of highest costs at about 2,825 ms after the response in trial n − 2 (although noise is added to this estimation due to differences in reaction times).
Rights and permissions
About this article
Cite this article
Scheil, J., Kleinsorge, T. Tracing the time course of n − 2 repetition costs in task switching. Exp Brain Res 232, 3535–3544 (2014). https://doi.org/10.1007/s00221-014-4044-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-014-4044-4