Abstract
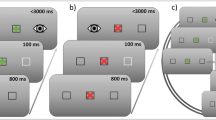
When we fixate an object, our eyes are not entirely still, but undergo small displacements such as microsaccades. Here, we investigate whether these microsaccades are sensitive to the preparatory processes involved in programming a saccade. We show that the frequency of microsaccades depends in a specific manner on the intention where to move the eyes (towards a target location or away from it), when to move (immediately after the onset of the target or after a delay), and what type of cue is followed (a peripheral onset or a centrally presented symbolic cue). In particular, in the preparatory interval before and early after target onset, more microsaccades were found when a delayed saccade towards a peripheral target was prepared than when a saccade away was programmed. However, no such difference in the frequency of microsaccades was observed when saccades were initiated immediately after the onset of the target or when the saccades were programmed on the basis of a centrally presented arrow cue. The results are discussed in the context of the neural correlates of response preparation, known as preparatory set.


Similar content being viewed by others
References
Basso MA (1998) Cognitive set and oculomotor control. Neuron 21:665–668
Basso MA, Wurtz RH (1997) Modulation of neuronal activity by target uncertainty. Nature 389:66–69
Basso MA, Wurtz RH (1998) Modulation of neuronal activity in superior colliculus by changes in target probability. J Neurosci 18:7519–7534
Betta E, Turatto M (2006) Are you ready? I can tell by looking at your microsaccades. Neuroreport 17:1001–1004
Bridgeman B, Palca J (1980) The role of microsaccades in high acuity observational tasks. Vis Res 20:813–817
Connolly JD, Goodale MA, Menon RS, Munoz DP (2002) Human fMRI evidence for the neural correlates of preparatory set. Nat Neurosci 5:1345–1352
DeSouza JF, Menon RS, Everling S (2003) Preparatory set associated with pro-saccades and anti-saccades in humans investigated with event-related FMRI. J Neurophysiol 89:1016–1023
Ditchburn RW, Ginsborg BL (1953) Involuntary eye movements during fixation. J Physiol 119:1–17
Engbert R, Kliegl R (2003) Microsaccades uncover the orientation of covert attention. Vis Res 43(9):1035–1045
Engbert R, Mergenthaler K (2006) Microsaccades are triggered by low retinal image slip. Proc Natl Acad Sci USA 103:7192–7197
Evarts E, Shinoda Y, Wise S (1984) Neurophysiological approaches to higher brain functions. Wiley, New York
Everling S, Munoz DP (2000) Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J Neurosci 20:387–400
Everling S, Dorris MC, Klein RM, Munoz DP (1999) Role of primate superior colliculus in preparation and execution of anti-saccades and pro-saccades. J Neurosci 19:2740–2754
Fecteau JH, Munoz DP (2003) Exploring the consequences of the previous trial. Nat Rev Neurosci 4:435–443
Galfano G, Betta E, Turatto M (2004) Inhibition of return in microsaccades. Exp Brain Res 159(3):400–404
Gandhi N, Keller E (1999) Activity of the brain stem omnipause neurons during saccades perturbed by stimulation of the primate superior colliculus. J Neurophysiol 82:3254–3267
Hafed ZM, Clark JJ (2002) Microsaccades as an overt measure of covert attention shifts. Vis Res 42:2533–2545
Hafed ZM, Goffart L, Krauzlis RJ (2009) A neural mechanism for microsaccade generation in the primate superior colliculus. Science 323:940–943
Hallett PE, Adams BD (1980) The predictability of saccadic latency in a novel voluntary oculomotor task. Vis Res 20:329–339
Hebb DO (1972) Textbook of physiology. Saunders, Philadelphia
Horowitz TS, Fine EM, Fencsik DE, Yurgenson S, Wolfe JM (2007) Fixational eye movements are not an index of covert attention. Psychol Sci 18:356–363
Krauzlis RJ, Basso MA, Wurtz RH (1997) Shared motor error for multiple eye movements. Science 276(5319):1693–1695
Laubrock J, Engbert R, Kliegl R (2005) Microsaccade dynamics during covert attention. Vis Res 45(6):721–730
Laubrock J, Engbert R, Rolfs M, Kliegl R (2007) Microsaccades are an index of covert attention: commentary on Horowitz, Fine, Fencsik, Yurgenson, and Wolfe (2007). Psychol Sci 18(4):364–366
Martinez-Conde S (2006) Fixational eye movements in normal and pathological vision. Prog Brain Res 154:151–176
Munoz DP, Everling S (2004) Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci 5:218–228
Munoz DP, Wurtz RH (1993a) Fixation cells in monkey superior colliculus I. Characteristics of cell discharge. J Neurophysiol 70(2):559–575
Munoz DP, Wurtz RH (1993b) Fixation cells in monkey superior colliculus II. Reversible activation and deactivation. J Neurophysiol 70:576–589
Rolfs M, Engbert R, Kliegl R (2004) Microsaccade orientation supports attentional enhancement opposite a peripheral cue: commentary on Tse, Sheinberg, and Logothetis (2003). Psychol Sci 15(10):705–707
Rolfs M, Engbert R, Kliegl R (2005) Crossmodal coupling of oculomotor control and spatial attention in vision and audition. Exp Brain Res 166:427–439
Rolfs M, Laubrock J, Kliegl R (2006) Shortening and prolongation of saccade latencies following microsaccades. Exp Brain Res 169:369–376
Rolfs M, Kliegl R, Engbert R (2008a) Towards a model of microsaccade generation: the case of microsaccadic inhibition. J Vis 8(11):1–23
Rolfs M, Laubrock J, Kliegl R (2008b) Microsaccade-induced prolongation of saccadic latencies depends on microsaccade amplitude. J Eye Mov Res 1(3):1–8
Schlag-Rey M, Amador N, Sanchez H, Schlag J (1997) Antisaccade performance predicted by neuronal activity in the supplementary eye field. Nature 390:398–401
Slagter HA, Weissman DH, Giesbrecht B, Kenemans JL, Mangun GR, Kok A et al (2006) Brain regions activated by endogenous preparatory set shifting as revealed by fMRI. Cogn Affect Behav Neurosci 6:175–189
Steinman RM, Cunitz RJ, Timberlake GT, Herman M (1967) Voluntary control of microsaccades during maintained monocular fixation. Science 155:1577–1579
Steinman RM, Haddad GM, Skavenski AA, Wyman D (1973) Miniature eye movement. Science 181:810–819
Theeuwes J, Kramer AF, Hahn S, Irwin DE, Zelinsky GJ (1999) Influence of attentional capture on oculomotor control. J Exp Psychol Hum Percept Perform 25:1595–1608
Tse PU, Sheinberg DS, Logothetis NK (2004) The distribution of microsaccade directions need not reveal the location of attention. Psychol Sci 15:708–710
Valsecchi M, Betta E, Turatto M (2007) Visual oddballs induce prolonged microsaccadic inhibition. Exp Brain Res 177(2):196–208
Walker R, Walker DG, Husain M, Kennard C (2000) Control of voluntary and reflexive saccades. Exp Brain Res 130:540–544
Zuber BL, Stark L, Cook G (1965) Microsaccades and the velocity-amplitude relationship for saccadic eye movements. Science 150(3702):1459–1460
Acknowledgments
This work was supported by The Leverhulme Trust (grant F-07537-Z) and the Economic and Social Research Council (ESRC; grant RES-000-22-2932). We would like to thank Martin Rolfs for his suggestions and comments on previous versions of this paper and the people in Potsdam for useful discussions on the experiments and the results.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hermens, F., Zanker, J.M. & Walker, R. Microsaccades and preparatory set: a comparison between delayed and immediate, exogenous and endogenous pro- and anti-saccades. Exp Brain Res 201, 489–498 (2010). https://doi.org/10.1007/s00221-009-2061-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-009-2061-5