Abstract
Spatial perception is sensitive to the energetic costs required to perform intended actions. For example, hills look steeper to people who are fatigued or burdened by a heavy load. Similarly, perceived distance is also influenced by the energy required to walk or throw to a target. Such experiments demonstrate that perception is a function, not just of optical information, but also of the perceiver’s potential to act and the energetic costs associated with the intended action. In the current paper, we expand on the notion of “cost” by examining perceived distance in patients diagnosed with chronic pain, a multifactorial disease, which is experienced while walking. We found that chronic pain patients perceive target distances to be farther away compared with a control group. These results indicate the physical, and perhaps emotional, costs of chronic pain affect spatial perceptions.
Similar content being viewed by others
Introduction
When people anticipate performing an effortful task, their spatial perceptions are influenced by the physiological demands associated with energy expenditure. Hills look steeper to people who are tired, encumbered, of low fitness, or elderly and in declining health (Bhalla and Proffitt 1999; Proffitt et al. 1995); and distances look farther to people following experimental manipulations associated with increased walking or throwing effort (Proffitt et al. 2003; Witt et al. 2004). We have argued that spatial perceptions relate the optically specified environment to the anticipated cost associated with performing actions in that environment. In previous experiments (Bhalla and Proffitt 1999), the cost was defined in terms of metabolic energy expenditure. From an evolutionary perspective, conserving energy is a survival imperative, and thus, there are good reasons for having a perceptual system that is sensitive to the energetic costs associated with acting within one’s environment (see Proffitt 2006).
An energetic cost represents a reduction in the organism’s potential to perform adaptive actions. However, if one interprets the concept of costs of behavior more broadly, it becomes apparent that there may be other influences on spatial perception beyond the energetic variety. The motivation for the current study was to extend the notion of behavioral costs by examining a group of individuals who suffer from chronic pain in the lower back and legs. Not surprisingly, patients with such pain are often reluctant to perform actions that produce uncomfortable and often excruciating sensations (Kori et al. 1990; Picavet et al. 2002). Thus, they experience physical and emotional costs associated with movement that might impact perception of spatial layout in a similar fashion to the energetic costs documented in earlier studies. To anticipate the key result, we found that patients suffering from chronic low back or leg pain did perceive visually presented targets to be farther away compared with a group of pain-free controls.
Methods
Participants
Ten patients diagnosed with benign chronic musculoskeletal and/or neuropathic pain of the low back and/or lower extremities were recruited at the pain management center (PMC) at the University of Virginia Health System (2 males, 8 females, mean age = 42.80). The clinical profile of this group is reported in Table 1. Eight employees (2 males, 6 females, mean age = 38.44) of the PMC and adjoining clinics served as control participants. All participants volunteered for the study and gave informed consent; no compensation was provided. The study was approved by the Institutional Review Board of the University of Virginia, and all participants were treated in accordance with the ethical principles of the American Psychological Association. All participants were naïve to the hypothesis of the experiment.
Materials and apparatus
The experiment took place in a long hallway carpeted in a pattern that did not provide obvious cues for estimating distance. Orange sports cones were used to mark the target distances and the participants’ location. Tape, which could not be seen from the subjects’ vantage point, was placed on the walls to mark the distances. An 18″ ruler was available for subjects to hold while estimating distance.
Procedure
Participants were approached by an experimenter after their medical appointments, during which they had been assessed on a variety of measures (see Table 1). They were asked if they would participate in an experiment on distance perception. Once consent was obtained an experimenter walked them out of the PMC and along a hallway heading toward the building’s main exit. The experimenter stopped the participants at a pre-marked point and explained that they would view a series of cones placed in the hallway at various distances from where they stood, and that they were to estimate the distance to each cone in feet and inches. We offered an 18″ ruler to use as a reference. Participants estimated the distance to cones placed at 4, 5, 7, and 9 m. Participants estimated the distance to targets at all four distances, and the order of distances randomized across participants. After participants estimated all four distances, they were shown a sample distance of either 6 or 8 m (chosen at random) and asked how many times they could walk back and forth over that distance before experiencing pain.
Results
Data from one patient were excluded from analysis based on a self-reported lack of pain during walking. The rest of the pain patients indicated that they would experience pain at least by the first time walking to the target and back. None of the controls said that they would feel any pain walking back and forth.
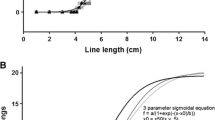
Distance estimates from one patient and one member of the control group were deemed outliers (patient outlier mean = 91.44 m, control outlier mean = 28.96 m, both were over 3 times the interquartile range of their respective groups) and excluded from analysis. The remaining data (N = 8 patients, mean age = 40.63; 7 controls, mean age = 38.79) were entered into a repeated-measures ANOVA with distance (4, 5, 7, and 9 m) as a within-subjects factor and group (controls vs. pain patients) as a between-subjects factor. There was a main effect of distance, F(3, 39) = 36.16, P < 0.001, η 2 = 0.73. Critically, there was also a significant main effect of group, F(1, 13) = 9.25, P < 0.01, η 2 = 0.42. As shown in Fig. 1, participants who experienced pain when walking perceived targets as farther away compared with pain-free controls. The interaction between distance and group was not significant, F(3, 39) = 1.87, P = 0.15.
Discussion
Patients who experienced chronic lower body pain when walking perceived the targets to be farther away than did pain-free controls. This finding is consistent with earlier studies documenting effects of action-related effort on perception of spatial layout and further supports the notion that individuals perceive the world in terms of the costs of acting within it (e.g. Proffitt et al. 2003; Witt et al. 2004). The biopsychosocial model of chronic pain (Flor and Hermann 2004) provides that this cost could include increased pain sensation as well as cognitive, emotional and social aspects of suffering.
Although the distance estimates of the chronic pain patients were, in fact, more accurate than were those of the controls’, this should not be interpreted as showing that chronic pain improves accuracy in perceived distance. This is because verbal judgments of distances in this range are generally underestimated (e.g. Loomis et al. 1992). Thus, chronic pain patients overestimated the distance to the targets relative to normal distance compression found in the pain-free controls.
A growing body of evidence indicates cognitive impairment in chronic pain (Kreitler and Niv 2007). Most chronic pain patients complain of cognitive difficulties, particularly with memory and attention, and objective testing supports some deficits in these and other cognitive domains including response speed and mental flexibility. However, we do not believe that cognitive impairment factored into our results. The variability was not significantly greater in our chronic pain sample than in our controls, suggesting a specific bias in perceived distance rather than a general cognitive impairment.
In addition to pain, other potential contributors may include depression, medication use (especially opioid analgesics), and sleep disturbances. Although the mean score in the pain group suggests a mild level of depression, the score of 21.7 is below the recommended cutoff for major depression in chronic pain patients (Geisser et al. 1997). Furthermore, there is no published evidence to suggest that depression of any severity affects distance perception. In fact, one study shows that depressed individuals do not perceive hills to be steeper than non-depressed individuals (Riener 2007).
All patients in our clinical sample were taking prescribed opioids. Sleep was not specifically assessed but is likely to have been disturbed for many of our patients given the prevalence of such problems in this population and our patients’ perceived disability ratings (which include sleep impairment). Although no research has investigated the relationship between sleep deprivation and perceived distance, fatigue from physical exertion of rock climbing influences people’s judgments of perceived maximum reaching height (Pijpers et al. 2007). Given the relationship now documented between perception and action capabilities (e.g. Witt et al. 2004, 2005), we would predict that factors that affected action abilities (e.g. medications, sleep deprivation) would also influence perception.
Future studies could take many directions. We would expect to see similar effects of pain on other aspects of spatial perception such as perceived geographic slant as long as the hill afforded ascending. We also expect the effects of pain on perceived distance to be specific to the sites of pain. We would predict that far distances such as the ones used here would not look as far to patients with chronic pain in their upper extremities. However, patients with pain in their upper extremities would likely perceive reachable distances to be farther than people without pain in their upper extremities. Recent research suggests that perceived distance in near space reflects one’s ability to act in that space. Targets within reach as a result of holding a tool look closer than when the tool is not used (Witt et al. 2005). Heavier objects presented within reach look farther away than lighter objects presented within reach (Linkenauger et al. 2008). Just like the energetic costs associated with reaching and grasping these objects, the costs associated with chronic pain involving the arms might also influence perceived distance to nearby objects.
An alternative possibility is that chronic pain results in an expansion of perceived distance in all spaces, not just in the space that affords painful actions. In other words, patients with chronic pain in the legs might perceive targets presented at shorter distances, specifically within the range of reaching, as farther compared to pain-free controls even though the patients do not have pain when reaching. Such a result would suggest more global influences of chronic pain on perceived distance, as opposed to perceptual effects that are specific to painful actions. The current study does not disentangle these possibilities. Previous research on pain-free participants demonstrates specificity related to the intended action. When walking to a target requires more effort, the target only looks farther away to people who intend to walk but not to people who intend to throw. Likewise, when throwing to a target requires more effort, the target only looks farther away to people who intend to throw but not to people who intend to walk (Witt et al. 2004). If pain’s influence on perceived distance is similarly action-specific, then we would expect that a distant target will look farther away to people who experience pain when walking but not to people who experience pain when, for example, reaching. Similarly, we would expect that when reaching to a target is painful, then a target within reach will look farther away to people who experience pain when reaching but not to people who experience pain when walking. Thus, we predict that the effects of chronic pain on perceived distance are specific to the actions during which the pain is experienced. Further research, however, will be required to determine whether pain’s influence on spatial perception is action-specific in this way.
In addition, with larger and more diverse samples of pain patients, correlations between pain type and/or etiology and perceived distance could be found. If this were the case, spatial perception could be evaluated as an additional diagnostic or classification tool for pain assessment. Medications used to treat chronic pain might also be evaluated for their impact on spatial perception.
This study also yields improved insight into the experience of chronic pain. While a distance may not seem far to someone who does not experience pain, that distance may look roughly 30% farther to someone with chronic lower body pain. We would expect this pattern to stay consistent or perhaps even increase with farther distances. Better understanding of the difficulties these patients experience in their daily lives is an important step toward helping them cope with persistent pain and for improving treatments. Results may also have important implications for established theoretical models of chronic pain and associated behaviors. For example, the fear-avoidance model (Leeuw et al. 2007) has been applied to demonstrate associations between pain, task avoidance, disability, and cognitive-emotional variables such as fear of pain/injury, catastrophizing, and pain vigilance. Spatial perception should be evaluated as another element of this cyclical model and a potential moderator of relationships between pain experience and avoidance behaviors.
References
Bhalla M, Proffitt DR (1999) Visual-motor recalibration in geographical slant perception. J Exp Psychol Hum Percept Perform 25:1076–1096
Flor H, Hermann C (2004) Biopsychosocial models of pain. In: Dworkin RH, Breitbart WS (eds) Psychosocial aspects of pain: a handbook for health care providers, progress in pain research and management, vol 28. IASP Press, Seattle, pp 283–304
Geisser ME, Roth RS, Robinson ME (1997) Assessing depression among persons with chronic pain using the center for epidemiological studies-depression scale and the beck depression inventory: a comparative analysis. Clin J Pain 13:163–170
Kori SH, Miller RP, Todd DD (1990) Kinesiophobia: a new view of chronic pain behavior. Pain Manag 3:35–43
Kreitler S, Niv D (2007) Cognitive impairment in chronic pain. Pain Clin Updates 15(4):1–4
Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JWS (2007) The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med 30:77–94
Linkenauger SL, Zadra J, Witt JK, Proffitt DR (2008) Effort affects perceived distance to objects within reach. University of Virginia, Charlottesville (in preparation)
Loomis JM, Da Silva JA, Fujita N, Fukusima SS (1992) Visual space perception and visually directed action. J Exp Psychol Hum Percept Perform 18:906–921
Picavet HS, Vlaeyen JW, Schouten JS (2002) Pain catastrophizing and kinesiophobia: predictors of chronic low back pain. Am J Epidemiol 156:1028–1034
Pijpers JR, Oudejans R, Bakker F (2007) Changes in the perception of action possibilities while climbing to fatigue on a climbing wall. J Sports Sci 25:97–110
Proffitt DR (2006) Embodied perception in the economy of action. Perspect Psychol Sci 1:110–122
Proffitt DR, Bhalla M, Gossweiler R, Midgett J (1995) Perceiving geographical slant. Psychon Bull Rev 2:409–428
Proffitt DR, Stefanucci JK, Banton T, Epstein W (2003) The role of effort in distance perception. Psychol Sci 14:106–113
Riener C (2007) An effect of mood on perception of slant. Unpublished doctoral dissertation, University of Virginia, Charlottesville
Witt JK, Proffitt DR, Epstein W (2004) Perceiving distance: a role of effort and intent. Perception 33:577–590
Witt JK, Proffitt DR, Epstein W (2005) Tool use affects perceived distance but only when you intend to use it. J Exp Psychol Hum Percept Perform 31:880–888
Acknowledgments
This research was supported by an NIH RO1MH075781-01A2 grant to Dennis Proffitt.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Witt, J.K., Linkenauger, S.A., Bakdash, J.Z. et al. The long road of pain: chronic pain increases perceived distance. Exp Brain Res 192, 145–148 (2009). https://doi.org/10.1007/s00221-008-1594-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-008-1594-3