Abstract
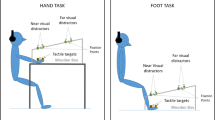
The body schema, a constantly updated representation of the body and its parts, has been suggested to emerge from body part-specific representations which integrate tactile, visual, and proprioceptive information about the identity and posture of the body. Studies using different approaches have provided evidence for a distinct representation of the visual space ~30 cm around the upper body, and predominantly the hands, termed the peripersonal space. In humans, peripersonal space representations have often been investigated with a visual–tactile crossmodal congruency task. We used this task to test if a representation of peripersonal space exists also around the feet, and to explore possible interactions of peripersonal space representations of different body parts. In Experiment 1, tactile stimuli to the hands and feet were judged according to their elevation while visual distractors presented near the same limbs had to be ignored. Crossmodal congruency effects did not differ between the two types of limbs, suggesting a representation of peripersonal space also around the feet. In Experiment 2, tactile stimuli were presented to the hands, and visual distractors were flashed either near the participant’s foot, near a fake foot, or in distant space. Crossmodal congruency effects were larger in the real foot condition than in the two other conditions, indicating interactions between the peripersonal space representations of foot and hand. Furthermore, results of all three conditions showed that vision of the stimulated body part, compared to only proprioceptive input about its location, strongly influences crossmodal interactions for tactile perception, affirming the central role of vision in the construction of the body schema.



Similar content being viewed by others
Notes
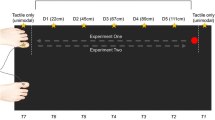
More specifically, for the 12 participants who performed both experiments, all conditions were presented intermixed and pseudo-randomized. However, because the foot condition of Experiment 1 required that the stimulators were attached to the feet (whereas for all other conditions they were attached to the hands), the foot condition was always run first or last.
To test if running Experiments 1 and 2 together (see Footnote 1) was responsible for the effects of the foot at the hidden hand, the results for the hidden hand were analyzed with an additional factor Group (participated in Experiment 1 [12 participants] vs. Experiment 2 Only [15 participants]). There was a marginally significant interaction in the comparison of the two groups (participants who performed both experiments vs. Experiment 2 Only), Group × Distractor Side × Distractor Congruency (F 1,25 = 4.03, P = 0.056). Whereas this interaction was not significant in the Experiment 2 Only group, it was significant for those participants who also performed Experiment 1 (F 1,11 = 16.55, P = 0.002). This was due to a greater difference between incongruent minus congruent conditions (i.e., the CCE) for same side distractors (159 ms) than for opposite side distractors (105 ms) in this group. In contrast, in the Experiment 2 Only group the CCE did not differ for the two sides (same side: 102 ms; opposite side: 101 ms). The fact that in Experiment 1 both stimulated limbs were always visible (and part of Experiment 1 was always run before any of the conditions of Experiment 2) may therefore have lead to a stronger distinction of same and opposite side distractors “belonging” to their respective side, leading to a reduced influence of opposite side distractors or an increased influence of same side distractors. In contrast, when participants never saw both stimulated limbs near the distractors, they do not appear to have coded the distractors as belonging to two sides for the side of the hidden hand. Finally, to see if this group effect was specific for the hidden hand, we examined the Group × Distractor Side × Distractor Congruency interaction also for the visible hand; here, this interaction was not significant (P > 0.25), confirming that, indeed, the group difference was related to the hidden hand only. Note that this distinction between the two groups does not affect the main conclusions of the results form Experiment 2, namely the interaction with factor Condition.
In the ANOVA comparing the two groups—those participants who participated in both Experiments 1 and 2 vs. those who only performed Experiment 2 (see Footnote 2)—none of the interactions involving Group and Condition were significant, with the exception of a just marginal interaction of Group × Condition × Distractor Side (F 2,50 = 2.50, P = 0.092), indicating that the two groups differed according to how same side vs. opposite side distractors influenced performance in the different conditions. In the group which also performed Experiment 1, the interaction of Condition × Distractor Side was not significant. In contrast, in the Experiment 2 Only group, this interaction was significant (F 2,28 = 4.57, P = 0.02); further analysis revealed that this was because participants responded slower for opposite side distractors overall in the fake foot condition, but not in the real foot and the empty compartment conditions. Note that this difference in the fake foot condition concerns responses to all stimuli of that side overall, and is unrelated to the CCE (incongruent vs. congruent distractors).
Abbreviations
- CCE:
-
Crossmodal congruency effect
- IE:
-
Inverse efficiency
- RF:
-
Receptive field
References
Duhamel JR, Colby CL, Goldberg ME (1998) Ventral intraparietal area of the macaque: congruent visual and somatic response properties. J Neurophysiol 79:126–136
Gallace A, Spence C (2005) Visual capture of apparent limb position influences tactile temporal order judgments. Neurosci Lett 379:63–68
Graziano MS, Cooke DF (2006) Parieto-frontal interactions, personal space, and defensive behavior. Neuropsychologia 44:845–859
Graziano MS, Hu XT, Gross CG (1997) Visuospatial properties of ventral premotor cortex. J Neurophysiol 77:2268–2292
Graziano MS, Reiss LA, Gross CG (1999) A neuronal representation of the location of nearby sounds. Nature 397:428–430
Graziano MS, Taylor CS, Moore T (2002) Complex movements evoked by microstimulation of precentral cortex. Neuron 34:841–851
Graziano MS, Yap GS, Gross CG (1994) Coding of visual space by premotor neurons. Science 266:1054–1057
Holmes NP, Calvert GA, Spence C (2004) Extending or projecting peripersonal space with tools? Multisensory interactions highlight only the distal and proximal ends of tools. Neurosci Lett 372:62–67
Holmes NP, Calvert GA, Spence C (2007) Tool use changes multisensory interactions in seconds: evidence from the crossmodal congruency task. Exp Brain Res 183:465–476
Holmes NP, Snijders HJ, Spence C (2006) Reaching with alien limbs: visual exposure to prosthetic hands in a mirror biases proprioception without accompanying illusions of ownership. Percept Psychophys 68:685–701
Holmes NP, Spence C (2004) The body schema and multisensory representation(s) of peripersonal space. Cogn Process 5:94–105
Iriki A, Tanaka M, Iwamura Y (1996) Coding of modified body schema during tool use by macaque postcentral neurones. Neuroreport 7:2325–2330
Kitagawa N, Spence C (2005) Investigating the effect of a transparent barrier on the crossmodal congruency effect. Exp Brain Res 161:62–71
Kitagawa N, Zampini M, Spence C (2005) Audiotactile interactions in near and far space. Exp Brain Res 166:528–537
Ladavas E (2002) Functional and dynamic properties of visual peripersonal space. Trends Cogn Sci 6:17–22
Maravita A, Spence C, Kennett S, Driver J (2002a) Tool-use changes multimodal spatial interactions between vision and touch in normal humans. Cognition 83:B25–B34
Maravita A, Spence C, Sergent C, Driver J (2002b) Seeing your own touched hands in a mirror modulates cross-modal interactions. Psychol Sci 13:350–355
Pavani F, Spence C, Driver J (2000) Visual capture of touch: out-of-the-body experiences with rubber gloves. Psychol Sci 11:353–359
Schicke T, Röder B (2006) Spatial remapping of touch: confusion of perceived stimulus order across hand and foot. Proc Natl Acad Sci USA 103:11808–11813
Shore DI, Spry E, Spence C (2002) Confusing the mind by crossing the hands. Brain Res Cogn Brain Res 14:153–163
Spence C, Kingstone A, Shore DI, Gazzaniga MS (2001) Representation of visuotactile space in the split brain. Psychol Sci 12:90–93
Spence C, Pavani F, Driver J (2004a) Spatial constraints on visual–tactile cross-modal distractor congruency effects. Cogn Affect Behav Neurosci 4:148–169
Spence C, Pavani F, Maravita A, Holmes N (2004b) Multisensory contributions to the 3-D representation of visuotactile peripersonal space in humans: evidence from the crossmodal congruency task. J Physiol Paris 98:171–189
World Medical Association (2004) Declaration of Helsinki (revised). http://www.wma.net/e/policy/b3.htm
Yamamoto S, Kitazawa S (2001) Reversal of subjective temporal order due to arm crossing. Nat Neurosci 4:759–765
Acknowledgments
We thank Sybille Röper for her help with data acquisition.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schicke, T., Bauer, F. & Röder, B. Interactions of different body parts in peripersonal space: how vision of the foot influences tactile perception at the hand. Exp Brain Res 192, 703–715 (2009). https://doi.org/10.1007/s00221-008-1587-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-008-1587-2