Abstract
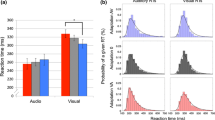
In a focused attention task saccadic reaction time (SRT) to a visual target stimulus (LED) was measured with an auditory (white noise burst) or tactile (vibration applied to palm) non-target presented in ipsi- or contralateral position to the target. Crossmodal facilitation of SRT was observed under all configurations and stimulus onset asynchrony (SOA) values ranging from −500 (non-target prior to target) to 0 ms, but the effect was larger for ipsi- than for contralateral presentation within an SOA range from −200 ms to 0. The time-window-of-integration (TWIN) model (Colonius and Diederich in J Cogn Neurosci 16:1000, 2004) is extended here to separate the effect of a spatially unspecific warning effect of the non-target from a spatially specific and genuine multisensory integration effect.














Similar content being viewed by others
Notes
This description of how the probability of integration and the probability of warning change with SOA should not conceal the assumption that, for any given trial, warning and integration cannot occur simultaneously.
FMINSEARCH uses the simplex search method of Lagarias et al. (1998).
There were also 2 “outlier” estimates beyond that range most likely due to some problem in the difficult optimization task.
References
Amlôt R, Walker R, Driver J, Spence C (2003). Multimodal visual-somatosensory integration in saccade generation. Neuropsychologia 41:1–15
Arndt A, Colonius H (2003) Two separate stages in crossmodal saccadic integration: evidence from varying intensity of an auditory accessory stimulus. Exp Brain Res 150:417–426
Bell AH, Corneil BD, Meredith MA, Munoz DP (2001) The influence of stimulus properties on multisensory processing in the awake primate superior colliculus. Can J Exp Psychol 55(2):123–132
Bell AH, Meredith A, Van Opstal AJ, Munoz DP (2005) Crossmodal integration in the primate superior colliculus underlying the preparation and initiation of saccadic eye movements. J Neurophysiol 93:3659–3673
Bushara KO, Grafman J, Hallett M (2001) Neural correlates of auditory-visual stimulus onset asynchrony detection. J Neurosci 21:300–304
Colonius H, Diederich A (2004) Multisensory interaction in saccadic reaction time: a time-window-of-integration model. J Cogn Neurosci 16:1000–1009
Corneil BD, Munoz DP (1996) The influence of auditory and visual distractors on human orienting gaze shifts. J Neurosci 16:8193–8207
Corneil BD, Van Wanrooij M, Munoz DP, Van Opstal AJ (2002). Auditory-visual interactions subserving goal-directed saccades in a complex scene. J Neurophysiol 88:438–454
Diederich A, Colonius H (2004) Modeling the time course of multisensory interaction in manual and saccadic responses. In: Calvert G, Spence C, Stein BE (eds) Handbook of multisensory processes. MIT, Cambridge
Diederich A, Colonius H (2007a) Why two “distractors” are better than one: modeling the effect of non-target auditory and tactile stimuli on visual saccadic reaction time. Exp Brain Res. doi:10.1007/s00221-006-0768-0
Diederich A, Colonius H (2007b) Modeling spatial effects in visual-tactile saccadic reaction time. Percept Psychophys 69(1):56–67
Diederich A, Colonius H, Bockhorst D, Tabeling S (2003) Visual-tactile spatial interaction in saccade generation. Exp Brain Res 148:328–337
Doyle MC, Walker R (2002) Multisensory interactions in saccade target selection: curved saccade trajectories. Exp Brain Res 142:116–130
Eimer M (2001) Crossmodal links in spatial attention between vision, audition, and touch: evidence from event-related brain potentials. Neuropsychologia 39:1292–1303
Frens MA, Van Opstal AJ (1998) Visual-auditory interactions modulate saccade-related activity in monkey superior colliculus. Brain Res Bull 46(3):211–224
Frens MA, Van Opstal AJ, Van der Willigen RF (1995) Spatial and temporal factors determine auditory-visual interactions in human saccadic eye movements. Percept Psychophys 57:802–816
Groh JM, Sparks DL (1996) Saccades to somotosensory targets. III. Eye-position-dependent somatosensory activity in primates superior colliculus. J Neurophysiol 75:439-453
Harrington LK, Peck CK (1998) Spatial disparity affects visual-auditory interactions in human sensorimotor processing. Exp Brain Res 122:247–252
Hublet C, Morais J, Bertelson P (1976) Spatial constraints on focussed attention: beyond the right side advantage. Perception 5:3–8
Hughes HC, Nelson MD, Aronchick DM (1998) Spatial characteristics of visual-auditory summation in human saccades. Vis Res 38:3955–3963
Jiang W, Wallace MT, Jiang H, Vaughan JW, Stein BE (2001) Two cortical areas mediate multisensory integration in superior colliculus neurons. J Neurophysiol 85:506–522
Jiang W, Jiang H, Stein BE (2002) Two corticotectal areas facilitate orientation behavior. J Cogn Neurosci 14:1240–1255
Kennett S, Eimer M, Spence C, Driver J (2001) Tactile-visual links in exogenous spatial attention under different postures: convergent evidence from psychophysics and ERPs. J Cogn Neurosci 13:462–478
Klein R, Kingstone A (1993) Why do visual offsets reduce saccadic latencies? Behav Brain Sci 16(3):583–584
Lagarias JC, Reeds JA, Wright MH, and Wright PE (1998) Convergence properties of the Nelder-Mead simplex method in low dimensions. SIAM J Optim 9(1):112–147
Lewald J, Guski R (2003) Cross-modal perceptual integration of spatially and temporally disparate auditory and visual stimuli. Cogn Brain Res 16:468–478
McDonald JJ, Teder-Sälejärvi WA, Hillyard SA (2000). Involuntary orienting to sound improves visual perception. Nature 407:906–908
Meredith MA (2002) On the neural basis for multisensory convergence: a brief overview. Cogn Brain Res 14:31–40
Meredith MA, Stein BE (1986a) Visual, auditory, and somatosensory convergence on cells in superior colliculus results in multisensory integration. J Neurophysiol 56:640–662
Meredith MA, Stein BE (1986b) Spatial factors determine the activity of multisensory neurons in cat superior colliculus. Brain Res 365:350–354
Meredith MA, Nemitz JW, Stein BE (1987) Determinants of multisensory integration in superior colliculus neurons. I. Temporal factors. J Neurosci 10:3215–3229
Morein-Zamir S, Soto-Faraco S, Kingstone A (2003) Auditory capture of vision: examining temporal ventriloquism. Cogn Brain Res 17:154–163
Navarra J, Vatakis A, Zampini M, Soto-Faraco S, Humphreys W, Spence C (2005) Exposure to asynchronous audiovisual speech extends the temporal window for audiovisual integration. Cogn Brain Res 25:499–507
Nickerson RS (1973) Intersensory facilitation of reaction time: energy summation or preparation enhancement. Psychol Rev 80:489–509
Posner MI (1980) Orienting of attention. Quart J Exp Psychol 32:3–25
Rach S, Diederich A (2006) Visual-tactile integration: Does stimulus duration influence the relative amount of response enhancement? Exp Brain Res 173:514–520
Reuter-Lorenz PA, Hughes HC, Fendrich R (1991) The reduction of saccadic latency by prior offset of the fixation point: an analysis of the gap effect. Percept Psychophys 49(2):167–175
Ross SM, Ross LE (1981) Saccade latency and warning signals: effects of auditory and visual stimulus onset and offset. Percept Psychophys 29(5):429–437
Schaefer M, Heinze HJ, Rotte M (2005) Viewing touch improves tactile sensory threshold. Neuroreport 16(4):367–70
Spence C, Driver J (1997) Audiovisual links in exogenous covert spatial orienting. Perc Psychophys 59(1):1–22
Spence C, Squire S (2003) Multisensory integration: maintaining the perception of synchrony. Curr Biol 13:R519–R521
Stein BE, Meredith MA (1993) The merging of the senses. MIT, Cambridge
Van Atteveldt NM, Formisano E, Blomert L, Goebel R (2006) The effect of temporal asynchrony on the multisensory integration of letters and speech sounds. Cereb Cortex. doi:10.1093/cercor/bhl007
Van Opstal AJ, Munoz DP (2004) Auditory-visual interactions subserving primate gaze orienting. In: Calvert G, SpenceC , Stein BE (eds) Handbook of multisensory processes. MIT, Cambridge, pp 373–393
Van Wassenhove V, Grant KW, Poeppel D (2007) Temporal window of integration in auditory-visual speech perception. Neuropsychologia 45:598–607
Wallace MT, Wilkinson LK, Stein BE (1996) Representation and integration of multiple sensory inputs in primate superior colliculus. J Neurophysiol 76:1246–1266
Wallace MT, Roberson GE, Hairston WD, Stein BE, Vaughan JW, Schirillo JA (2004) Unifying multisensory signals across time and space. Exp Brain Res 158:252–258
Ward LM (1994) Supramodal and modality-specific mechanisms for stimulus-driven shifts of auditory and visual attention. Can J Exp Psychol 48:242–259
Whitchurch EA, Takahashi TT (2006) Combined auditory and visual stimuli facilitate head saccades in the barn owl (Tyto alba). J Neurophysiol 96:730–745
Acknowledgment
This research was supported by grants from Deutsche Forschungsgemeinschaft Di 506/8-1 and /-3. We are grateful to Rike Steenken, and Stefan Rach for several discussions of this work and and to Dr. Annette Schomburg for her help in setting up the experiment.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
Here we derive the probability of the events I (multisensory integration) and W (warning) under the exponential distribution assumption. Specifically, the probability distributions for peripheral processing times V for a visual target and A for an auditory non-target are, respectively,
for t ≥ 0, and f V(t) = f A (t) ≡ 0 for t < 0. The corresponding distribution functions are referred to by F V(t) and F A (t).
Probability of integration: P(I)
By definition,
where τ denotes the SOA value and ω is the width of the integration window. Computing the integral expression requires that we distinguish between three cases for the sign of τ + ω:
-
(a)
τ< τ + ω < 0
$$ \begin{aligned} P(I) = & \int\limits_{-\tau - \omega}^{-\tau} \lambda_A {\rm e}^{-\lambda_A a} \{1-{\rm e}^{-\lambda_{\rm V}(a+\tau+\omega)}\} {\rm d}a \\ & + \int\limits_{-\tau}^\infty \lambda_A {\rm e}^{-\lambda_A a}\{{\rm e}^{-\lambda_{\rm V}(a+\tau)}-{\rm e}^{-\lambda_{\rm V}(a+\tau+\omega)}\} {\rm d}a \\ = & \frac{\lambda_{\rm V}}{\lambda_{\rm V}+\lambda_A}\; {\rm e}^{\lambda_A \tau}(-1+{\rm e}^{\lambda_A \omega}); \end{aligned} $$ -
(b)
τ < 0 < τ + ω
$$ \begin{aligned} P(I) = & \int\limits_0^{-\tau} \lambda_A {\rm e}^{-\lambda_A a} \{1-{\rm e}^{-\lambda_{\rm V}(a+\tau+\omega)}\} da \\ & + \int\limits_{-\tau}^\infty \lambda_A {\rm e}^{-\lambda_A a}\{{\rm e}^{-\lambda_{\rm V}(a+\tau)}-{\rm e}^{-\lambda_{\rm V}(a+\tau+\omega)}\} da \\ = & \frac{1}{\lambda_{\rm V}+\lambda_A}\; \{\lambda_A(1-{\rm e}^{-\lambda_{\rm V}(\omega+\tau)})+\lambda_{\rm V}(1-{\rm e}^{\lambda_A \tau})\}; \end{aligned} $$ -
(c)
0 < τ < τ + ω
$$ \begin{aligned} P(I) = & \int\limits_0^\infty \lambda_A {\rm e}^{-\lambda_A a}\{{\rm e}^{-\lambda_{\rm V}(a+\tau)}-{\rm e}^{-\lambda_{\rm V}(a+\tau+\omega)}\} {\rm d}a \\ & = \frac{\lambda_A}{\lambda_{\rm V}+\lambda_A}\; \{{\rm e}^{-\lambda_{\rm V}\tau}-{\rm e}^{-\lambda_{\rm V} (\omega+\tau)}\}. \end{aligned} $$
Probability of warning: P(W)
By definition,
Again, we need to consider different cases:
-
(i)
τ + γ A < 0
$$ \begin{aligned} P(W) = & 1- \int\limits_{-\tau-\gamma_A}^\infty \lambda_A {\rm e}^{-\lambda_A a}\{1-{\rm e}^{-\lambda_{\rm V}(a+\tau+\gamma_A)}\} {\rm d}a \\ = & 1- \frac{\lambda_{\rm V}}{\lambda_{\rm V}+\lambda_A}\; {\rm e}^{\lambda_A(\tau + \gamma_A)}; \end{aligned} $$ -
(ii)
τ + γ A ≥ 0
$$ \begin{aligned} P(W) = & 1- \int\limits_{0}^\infty \lambda_A {\rm e}^{-\lambda_A a}\{1-{\rm e}^{-\lambda_{\rm V}(a+\tau+\gamma_A)}\} {\rm d}a \\ & = \frac{\lambda_A}{\lambda_{\rm V}+\lambda_A}\; {\rm e}^{-\lambda_{\rm V}(\tau + \gamma_A)}. \end{aligned} $$
Appendix 2
Tables 5, 6, 7, 8, 9 and 10 for mean SRTs under all uni- and bimodal conditions are listed, by participant.
Rights and permissions
About this article
Cite this article
Diederich, A., Colonius, H. Crossmodal interaction in saccadic reaction time: separating multisensory from warning effects in the time window of integration model. Exp Brain Res 186, 1–22 (2008). https://doi.org/10.1007/s00221-007-1197-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-007-1197-4